-
-
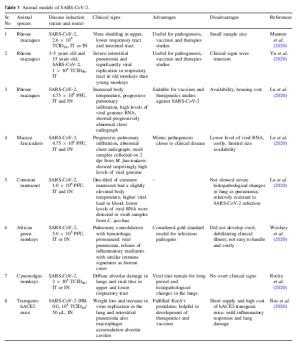
Sr. No Animal species Disease induction (strain and route) Clinical signs Advantages Disadvantages References 1 Rhesus macaques SARS-CoV-2, 2.6 × 106 TCID50, IT or IN Virus shedding in upper, lower respiratory tract and intestinal tract Useful for pathogenesis, vaccines and therapies studies Small sample size Munster et al. (2020) 2 Rhesus macaques 3–5 years old and 15 years old, SARS-CoV-2, 1 × 106 TCID50, IT Severe interstitial pneumonia and significantly viral replication in respiratory tract in old monkeys than young monkeys Useful for pathogenesis, vaccines and therapies studies Clinical signs were transient Yu et al. (2020) 3 Rhesus macaques SARS-CoV-2, 4.75 × 106 PFU, IT and IN Increased body temperature, progressive pulmonary infiltration, high levels of viral genome RNA, showed progressively abnormal chest radiograph Suitable for vaccines and therapeutics studies against SARS-CoV-2 Availability, housing cost Lu et al. (2020) 4 Macaca fascicularis SARS-CoV-2, 4.75 × 106 PFU, IT and IN Progressive pulmonary infiltration, abnormal chest radiograph; swab samples collected on 2 dpi from M. fascicularis showed surprisingly high levels of viral genome Mimic pathogenesis closer to clinical disease Lower level of viral RNA, costly, limited size availability Lu et al. (2020) 5 Common marmoset SARS-CoV-2, 1.0 × 106 PFU, IT and IN One-third of common marmoset had a slightly elevated body temperature, higher viral load in blood, lower levels of viral RNA were detected in swab samples from C. jacchus – Not showed severe histopathological changes in lung as pneumonia, relatively resistant to SARS-CoV-2 infection Lu et al. (2020) 6 African green monkeys SARS-CoV-2, 5.0 × 105 PFU, IT or IN Pulmonary consolidation with hemorrhage, pronounced viral pneumonia, release of inflammatory mediators with similar immune signatures as human cases Considered gold standard model for infectious pathogens Did not develop overt, debilitating clinical illness; not easy to handle and costly Woolsey et al. (2020) 7 Cynomolgus monkeys SARS-CoV-2, 2 × 105 TCID50, IT or IN Diffuse alveolar damage in lungs and viral titer in upper and lower respiratory tract Viral titer remain for long period and histopathological changes in the lungs No overt clinical signs Rockx et al. (2020) 8 Transgenic hACE2 mice SARS-CoV-2 (HB-01), 105 TCID50/50 μL, IN Weight loss and increase in virus replication in the lung and interstitial pneumonia also macrophages accumulation alveolar cavities Fulfilled Koch's postulates; helpful in development of therapeutics and vaccines Short supply and high cost of hACE2-transgenic mice; mild inflammatory responses and lung damage Bao et al. (2020) 9 BALB/c mice SARS-CoV-2 (MACSp6), 7.2 × 105 PFU, IN Infected all ages of mice; acute inflammatory responses closely related to the damage of lung tissues; levels of chemokines increased significantly in the aged mice as comparison to younger mice Easy handling breeding, convenient, economical, and effectively used for evaluation of in vivo evaluation of vaccines and therapeutics Exhibited moderate inflammatory responses Gu et al. (2020) 10 BALB/c mice 10-week old and 12 month-old SARS-CoV-2 MA, 105 PFU, IN Age-related increase in pathogenesis Useful for pathogenesis, vaccine immunogenicity and therapeutic efficacy studies – Dinnon et al. (2020) 11 Transgenic hACE2 mice HFH4-hACE2 in C3B6 mice) SARS-CoV-2, 3 × 104 TCID50 (for naïve infection) or 7 × 105 TCID50 (for the viral challenge), IN Weight loss, interstitial pneumonia, lymphopenia, gender susceptibility, viral titer in eye, heart & brain apart from lungs Partially simulated COVID-19 pathology LD50 of the model is not determined; lethal encephalitis Jiang et al. (2020) 12 hACE2 mice SARS-CoV-2 4 × 105 PFU-IN, 4 × 106 PFU-IG High viral titre in lung, brain and trachea; interstitial pneumonia; increase cytokines levels Helpful in study of transmission, pathogenesis, evaluating of vaccines and therapeutic efficacy - Sun et al. 2020b 13 Ad5-hACE2-transduced mice SARS-CoV-2 1 × 105 PFU-IN Weight loss, high virus titer in lungs, severe pulmonary pathology Useful for the study of pathogenesis and testing for antiviral therapeutics and vaccines Absence of critical condition and extra-pulmonary manifestations of infection Sun et al. 2020a 14 HACE2-transduced mice SARS-CoV-2 1 × 105 FFU- IN & IV Weight loss, high viral loads in lung, severe lung pathology Helpful to study pathogenesis, vaccines and therapeutics Mouse to mouse variation in expression of hACE2, tissue distribution and mild bronchial Hassan et al. 2020 15 Golden Syrian hamster SARS-CoV-2, 105–107 TCID50, IN Rapid breathing, loss of weight, diffuse alveolar damage and high lung viral load was observed Readily available, physiological, and highly similarity with COVID-19 useful for study of pathogenesis, therapeutics and vaccines There was a different outcomes in this study as comparison to previous study of SARS-CoV, not tested protein expression only tested mRNA of the hamsters cytokine profiles Chan et al. (2020) 16 Golden Syrian hamster SARS-CoV-2, Beta-CoV/Hong Kong/VM20001061/2020 virus, 8 × 104 TCID50, IN Weight loss, significant viral replication, transmission of infection via aerosols Useful for immunological studies for vaccine development Rapid viral clearance on 7 dpi Sia et al. (2020) 17 Ferrets SARS-CoV-2, 105.5 TCID50, IN Showed increased body temperatures and high virus titers in upper respiratory tracts Viral infection and transmission Low viral titer in lungs Kim et al. (2020) SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IN, intranasal; IT, intratracheal; IG, intragastric; TCID50, 50% Median Tissue Culture Infectious Dose; PFU, Plaque forming units; dpi, day post infection Table 1. Animal models of SARS-CoV-2.
-
Sr. No Animal model Disease induction (strain & route) Clinical signs Advantages Disadvantages References 1 Rhesus macaques SARS-CoV, Tor2, 107 PFU, IV/IT Significant viral titer in the lungs Useful for therapeutic evaluation and vaccines immunogenicity In SARS study limited use Rowe et al. (2004) 2 Rhesus macaques SARS-CoV, Urbani, 106 PFU, IT/ IN Could not show any clinical sign Used for Therapeutic evaluation and vaccines immunogenicity Clinical illness was not present McAuliffea et al. (2004) 3 Rhesus macaques SARS-CoV, PUMC01, 105 PFU, IN Pulmonary changes were observed on 5–60 dpi; All macaques reported fever 2–3 dpi Immunological and pathological similarity with clinical condition The symptoms are less severe as comparison of clinical scenario Qin et al. (2005) 4 Cynomolgus monkeys SARS-CoV, 103 and 106 TCID50, IN/IT Interstitial pneumonia, alveolar macrophages and neutrophils, diffuse alveolar damage Helpful for the study of SARS pathogenesis and can be used for therapeutic and vaccine studies Availability and cost Fouchier et al. (2003) 5 Cynomolgus monkeys SARS-CoV, 1 × 106 TCID50, IT/ IN, Symptoms appeared as difficulty in respiration; more diffuse alveolar damage, type 2 pneumocyte hyperplasia and alveolar macrophages are present in alveolar lumina Useful for vaccine and therapeutic drug evaluation There is issue with availability, housing cost; early clearance of virus and pneumonitis occurred Kuiken et al. (2003) 6 Cynomolgus monkeys SARS-CoV, Tor2, 107 PFU, IT Mild cough; a few scattered pleural adhesions Presentation of critical disease Symptoms cleared quickly and animals becomes asymptomatic from 8 to 10 days Rowe et al. (2004) 7 Cynomolgus monkeys SARS-CoV, Urbani, 3 × 106.3 PFU, IB/ IN, Reported nasal congestion, mild respiration distress; animals become lethargic; pulmonary related disease Helpful in immunogenicity of vaccines and therapeutics studies Lack of apparent clinical illness McAuliffe et al. (2004) 8 African green monkey SARS-CoV, Urbani, 106.3 TCID50, IB /IN, Virologic data and histopathologic findings, focal interstitial pneumonitis was noticed in some African green monkey Useful for evaluation of vaccine efficacy against infection Rapid clearance of virus and pneumonitis in African green monkey McAuliffe et al. (2004) 9 Marmoset SARS-CoV, Urbani, 106 PFU, IT Marmoset reported there is elevation of temperature, interstitial pneumonitis, multifocal lymphocytic hepatitis and diffuse interstitial colitis Can be used in Pathogenesis, therapeutics and vaccine efficacy studies This model is not able to explain the viral antigen/viral RNA within hepatic tissues Greenough et al. (2005) 10 BALB/c mice 4–6 week aged SARS-CoV, Urbani,
105 TCID50, INHigh viral load in respiratory tract Useful for immunological studies No overt clinical sign was present Subbarao et al. (2004) 11 BALB/c mice 12–14 month aged SARS-CoV, Urbani, 105 TCID50, IN Old aged mice observed significant loss of weight, mild dehydration and alveolar damage; Also reported intra-alveolar edema, perivascular infiltrates Useful for vaccine evaluation There is need of further characterization and immune senescence Vogel et al. (2007) 12 129SvEv /STAT 1-/- mice SARS-CoV, Tor2, 6 × 106 PFU/30 µL, IN Viral replication, morbidity, mortality and pneumonitis STAT 1-/- exhibited innate immunity Defect in innate immunity Hogan et al. (2004) 13 129SvEv /STAT 1-/- mice SARS-CoV, Urbani, 105 PFU, IN Reported severity of disease in this study, high virus replication and severe pulmonary lesions Viral replication, histopathology similar to clinical conditions Lack of a normal innate immune did not enhance virus pathogenesis Frieman et al. (2010) 14 C57BL/6 mice SARS-CoV, Urbani 1 × 104 TCID50, IN Virus infected the respiratory tract (bronchial and bronchiolar epithelium); failure to thrive and also observed infection reached to the brain Useful for immunological studies Fail to show clinical signs of disease Glass et al. (2004) 15 Transgenic hACE2 mice SARS-CoV, PUMC01 105 TCID50, IN Transgenic mice showed severe lung damage, systemic inflammatory reactions, degeneration, and necrosis in many extra-pulmonary organs More susceptible to infection as comparison to wild type mice, and more closely resemblance to human pathology of SARS Tissue distribution was limited, decreased expression of hACE2 and lack of lethality Yang et al. (2007) 16 Golden Syrian hamster 5-week aged, SARS-CoV, Urbani, 103TCID50, IN Increased viral replication in lungs, related interstitial pneumonitis, diffuse alveolar damage Reproducibility, increased viral replication in lungs which makes it suitable for immunoprophylaxis and immunotherapy and vaccines studies As virus is cleared rapidly from 7 to 10 dpi, there was no overt clinical illness Roberts et al. (2005) 17 Ferret and domestic cat SARS-CoV, 106 TCID50, IT Lethargy and mortality in ferrets; histopathological changes including pulmonary consolidation was reported in both animals Useful for vaccines, immunotherapy and therapeutic studies Availability; vulnerability to other respiratory viruses Martina et al. (2003) SARS, Severe acute respiratory syndrome; IN, intranasal inoculation; IT, intratracheal inoculation; IV, intravenous inoculation; IB, intrabronchial inoculation; TCID50, 50% Median Tissue Culture Infectious Dose; PFU, Plaque forming units. Table 2. Animal models of SARS-CoV.
-
Sr. No Animal model Disease induction (strain and route) Clinical Sign Advantage Disadvantage References 1 Dromedary camels HCoV-EMC/2012, 1 × 107 TCID50, IN, IT, CON Rhinorrhea; mild elevation in body temperature; nasal discharge; mild to moderate acute intraepithelial & sub-mucosal inflammation Reservoir source of the MERS-CoV Mild clinical symptoms Adney et al. (2014) 2 Alpacas Dromedary MERS-CoV Al-Hasa_KFU-HKU13/2013, 1 × 106 TCID50, Oronasal Positive deep nasal swab at 10 dpi Alpacas as a potential substitute for camels Small sample size; insufficient observation period of 21 days before rechallenge; mechanism not evaluated; Crameri et al. (2016) 3 Rhesus macaque HCoV-EMC/2012, 7 × 106 TCID50, IT, IN, oral, and ocular routes Virus shedding via nose, transient LRT infection Susceptible to infection Small sample size; transient model; no mortality de Wit et al.(2013a, b) 4 Rhesus macaques hCoV-EMC, 6.5 × 107 TCID50, IT Increased temperature on 1–2 dpi; multifocal mild-to-moderate interstitial pneumonia; types Ⅰ and Ⅱ pneumocytes and alveolar macrophages contain infection Susceptible to infection No detectable virus titers Yao et al. (2014) 5 Rhesus monkeys icMERS-0, 5 × 106 PFU, IT Lung hyperdensity changes, minimal-to-mild interstitial pneumonia; transient, mild pulmonary pathology High replication of icMERS-0 Mild infection Cockrell et al. (2018) 6 Rhesus macaques (immunosupressive) EMC/2012, 7 × 106 TCID50, ocular High viral shedding; mild pulmonary pathology Immunopathogenic component Use of immunosuppressive drug is not clinically relevant; immunosuppressive group is not challenged; Prescott et al. (2018) 7 Common Marmoset HCoV-EMC/2012, 4 × 106 TCID50, IN, IT and ocular Loss of appetite; decreased levels of activity; progressive severe pneumonia; extensive lesions in the lungs; severe, partially lethal disease model Severe; longer duration; high viral loads in lungs; high viral load both in lower respiratory tract and in blood Small sample size; lack of additional control animals; subjects euthanized before the disease course could resolve Falzarano et al. (2014) 8 Common Marmoset MERS-CoV-Jordan-n3/2012, 5 × 107 PFU, IT; MERS-CoV-EMC/2012, 5 × 107 PFU, IT Respiratory rate increased; interstitial multifocal to coalescing moderate pneumonia – No systemic clinical symptoms; limited clinical signs; Johnson et al. (2015) 9 Yorkshire Landrace pigs MERS-CoV, 1 × 107 TCID50, IN Viral RNA detected till 7 dpi Inexpensive and easy to obtain No animal to animal transmission Vergara-Alert et al. (2017) 10 New Zealand white rabbits EMC/2012, 1 × 103 TCID50, or 1 × 105 TCID50, IV No clinical sign even on exposure to re-infection; Inexpensive and easy to obtain Studied asymptomatic infection, non-lethal infections, transient dose-dependent pulmonary infection which is not associated with clinical symptoms Houser et al. (2017) 11 Transgenic mice (Ad5-hDPP4) EMC-2012, 1 × 105 PFU, IN Weight loss, virus replication in respiratory tract, interstitial pneumonia Useful in screening of therapeutics and vaccines efficacy; Efficient and rapid generation of the model within 2–3 weeks; can be used in genetically deficient mice Level of expression, tissue distribution Zhao et al. (2014) 12 Trangenic C57BL/6 J mice(hCD26/DPP4) CAGG enhancer/promoter, intron sequence, human CD26 cDNA, and rabbit beta globin poly(A) signal EMC-2012, 1 × 106 TCID50, IN Increase virus titers in lungs and brains; progressive pneumonia; characterized by extensive; significant mortality Susceptible to the infection Severe morbidity and mortality; hinders investigations of underlying mechanism Agrawal et al. (2015) 13 Swiss Webstar mice (hDPP4) (VelociGene technology) MERS-CoV- Hu/Jordan-N3/2012, 2 × 105 PFU, IN Mild–moderate peribronchiolar & alveolar inflammation Rapid mouse model production; no prior transduction is required; No cerebral inflammation Pascal et al. (2015) 14 Trangenic C57BL/6 J mice(hDPP4 model) (hCD26/DPP4) CAGG enhancer/promoter, intron sequence, human CD26 cDNA, and rabbit beta globin poly(A) signal EMC-2012, 1 × 106 TCID50, IN Persistent inflammatory infiltrates in the lungs; focal infiltrates in brain & liver Fully permissive to viral infection Severe morbidity and mortality; hinders investigations of underlying mechanism Tao et al. (2015) 15 Transgenic C57BL/6 mouse (hDPP4) model (purified 5861 bp fragment generated from ApaL1 digestion of pCAGGS-hDPP4) HCoV-EMC/2012 strain, 1 × 104.3 TCID50, IN Decreased activity; significant weight loss; mortality Mimic severe MERS pathology Underlying mechanism; mechanism of lethal which was of aberrant inflammatory response was not studied Zhao et al. (2015) 16 Transgenic C57BL/6 J mice (CRISPR–Cas9 gene editing (homozygous & heterozygous strain)) HCoV-EMC/2012, Camel MERS, icMERS, MERS-0 at 5 × 105 PFU, IN; MERS-15 at 5 × 106 PFU, IN Mortality and haemorrhage at 6 dpi; 25%–30% weight loss; diffuse alveolar damage and severe respiratory disease; decreased pulmonary function as measured by plethysmography No confounding effects of CNS infection on mortality Use of high viral load Cockrell et al. (2016) 17 Transgenic C57BL/6 mice (hDPP4-KI mice, mouse Dpp4 exons 10–12 replaced with the human DPP4 codons) Plaque-purified MERSMA, 1 × 104 to 1 × 106 PFU, IN Diffuse alveolar damage; innate immune responses to infection; leukocyte response to infection Model does not require prior sensitization Pathological changes associated with species specific MERSMA mutation Li et al. (2017) 18 Transgenic C57BL/6 mouse (R26-hDPP4 mice, insert hDPP4 into the Rosa26 locus using CRISPR/Cas9) hCoV-EMC, 1.5 × 105 PFU, IN Severe ARDS; viral titer in CNS Specificity of the infection in lungs & in CNS Not severe or lethal Fan et al. (2018) 19 Transgenic mice on a C57BL/6 background express hDPP4 HCoV-EMC 2012 strain, 1 × 105 TCID50, IN Viral titer in lungs Age related immunopathology No severe disease and lethal infection, no nasal and brain inflammation; mild infection; Iwata-Yoshikawa et al. (2019) MERS, Middle east respiratory syndrome; IN, intranasal; IT, intratracheal; CON, Conjuctivital; TCID50, 50% Median Tissue Culture Infectious Dose; PFU, Plaque forming units; LRT, lower respiratory tract; ARDS, acute respiratory distress syndrome; CNS, central nervous system; dpi, day post infection. Table 3. Animal models of MERS-CoV.
Figure 1 个
Table 3 个