-
-
-

-
-
Order Year Country Gene access number Co-infection References 1 2009 Sweden FJ872544 PCV-2, TTV Blomström et al. (2009) 2 2010 China GU556573 to GU556591 PCV-2, TTV-1, TTV-2, CSFV Zhai et al. (2010) 3 2010 China HM053693 and HM053694 PKV, PCV, Frog virus Cheng et al. (2010) 4 2010 USA GQ387500 and GQ387499 PCV-2 Cheung et al. (2010) 5 2010 Ireland JF512472 and JF512473 Porcine adenovirus, Enteroviruses, Reoviruses, Circovirus, Porcine parvovirus McKillen et al. (2011) 6 2011 Ireland JF512472 and JF512473 ND McNair et al. (2011) 7 2011 China JF429834, JF429836 ND Lau et al. (2011) 8 2011 China HQ223038 ND Zeng et al. (2011) 9 2011 China GU902971 ND Shan et al.(2011a, b) 10 2011 Romania JF721404–JF721421 PCV-2 (data not shown) Cadar et al. (2011) 11 2012 China NA PEDV, PKV, RVA, TGEV Zhang et al. (2013) 12 2012 Hungary JN400850 to JN400879 PPV2, PPV3, PPV4, PBoV1, PBoV2, 6 V and 7 V and PCV-2 Csagola et al. (2012) 13 2013 Croatia KC701291–KC701314, KC687097– KC687100, KC701315–KC701332, KC701333– KC701356, KC767891 ND Cadar et al. (2013) 14 2013 Cameroon JX869077 to JX869100 Mixed infection of different groups of porcine parvovirus Ndze et al. (2013) 15 2013 Uganda JX854557 TTSuV1 and TTSuV2 Blomstrom et al. (2013) 16 2013 Korea KF425330 to KF425337
KF728243 to KF728248Co-infection between group1, 2 and 3 Choi et al. (2014) 17 2014 Slovakia and Czech Republic NA PCV-2, TTSuV1, TTSuV2, PBoV, PRRSV, PTV Vlasakova et al. (2014) 18 2015 North America KR709262 to KR709268 PRRSV Schirtzinger et al. (2015) 19 2015 Thailand AB973315-AB973334, AB973335-AB973354 PPV1, 2, 3, 4 Saekhow and Ikeda (2014) 20 2015 Japan LC090199 ND Zhang et al. (2016) 21 2016 Germany KU311698 Mycoplasma hyorhinis Pfankuche et al. (2016) 22 2017 Belgium KY426738 to KY426752 PAstV, PEDV, Porcine enterovirus, Porcine picobirnavirus Conceicao-Neto et al. (2017) 23 2018 Malaysia KX686996 to KX686700 PCV-2/PMWS Jacob et al. (2018) Table 1. General information of documented porcine bocavirus cases.
-
Tissue Key findings References Lymph nodes The infection rates of PBoV in the PMWS-affected pigs were twice higher than in the non-PMWS affected pigs. The co-infection of PBoV along with the TTSV and PCV-2 might have facilitate the development of PMWS Blomström et al. (2009) The co-existence of two bocavirus strain within the same fecal sample revealing inter and intra host genetic diversity Lau et al. (2011) The genetic diversity of the circulating bocavirus strains in Xinjiang belong to three subgroups of three different genetic groups Meng et al. (2018) Gastrointestinal tract The prevalence rate of PBoV was higher in stool samples and these viruses multiply in the intestinal tract of piglets Cheng et al. (2010) Respiratory tract The first evidence of infection of weaning piglets with respiratory tract symptoms representing an emerging virus for swine respiratory tract diseases Zhai et al. (2010) Nasopharyngeal sample PBoV were higher in nasopharyngeal samples in deceased pigs than in healthy pigs Lau et al. (2011) Mesenteric lymph nodes The mesenteric lymph node had the highest detection rate suggesting the pathogenesis of PBoV infection involves the lymphoid tissues Jacob et al. (2018) Inguinal lymph nodes 25% of the organ tested were positive for PBoV Jacob et al. (2018) Spleen 23.5% of the spleen tested were positive for PBoV Jacob et al. (2018) Tonsil Out of 80 tonsil samples, 23 samples were positive for the PBoV Saekhow and Ikeda (2014) The tonsil had the second highest detection rate suggesting the pathogenesis of PBoV infection involves the lymphoid tissues Jacob et al. (2018) Lung Porcine parvovirus 4 was similar to PBoV. After inoculation of tissue homogenate in the colostrum deprived piglets, clinical symptoms were observed. But due to coinfection with PCV-2. It was not clear whether PPV4 can cause disease on its own or contributed to the disease phenomenon Cheung et al. (2010) One lungs tissue sample was positive for the PBoV without coinfection of the PCV-2, suggesting PBoV as not the risk factor the Hungarian pigs Csagola et al. (2012) The first description of the prevalence of PBoV in Korean swine herds with the mean positive rate of 34.9% Choi et al. (2014) 33.3% of lungs tissues were positive for PBoV Jacob et al. (2018) Kidney PBoV was detected from kidney tissues of two pigs suggesting the ability of virus to replicate within kidney cells causing renal pathology Jacob et al. (2018) Cerebral tissue By using fluorescent in situ hybridization for histologic detection of encephalomyelitis assigns a potential role of PBoV in provoking CNS lesions Pfankuche et al. (2016) Liver 25% of liver tissue were positive for PBoV Jacob et al. (2018) Table 2. Tissue tropism of porcine bocavirus.
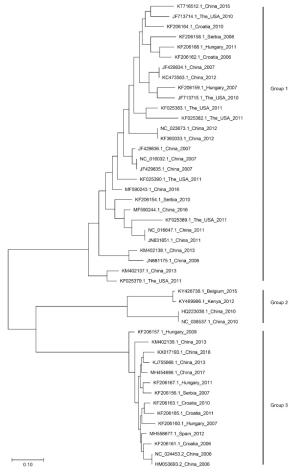
Figure 4 个
Table 2 个