-
-
-
No. Manufacture, organization name Test name Test type Gene or region detected Sample source Limits of detection Test result time/additional information Throughput information Country of approval 1 Shanghai ZJ Bio-Tech Co., Ltd. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real-time RT-PCR ORF1ab, E and N gene Throat swab, sputum, and BALF 1000 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400057) 1/26/2020, WHO, CE 2 Shanghai GeneoDX Biotech Co., LTD Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real-time RT-PCR ORF1ab and N gene Nasopharyngeal swab and BALF 500 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400058) 1/26/2020 3 BGI Biotechnology (Wuhan) CO., LTD Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real-time RT-PCR ORF1ab Throat swab and Bronchoalveolar Lavage Fluid (BALF) samples 100 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400060) 1/26/2020/CE Marked/FDA Authorized/PMDA Approved 4 Daan Gene Co., Ltd. of Sun Yat-Sen University 2019 Novel Coronavirus (2019-nCOV) RNA Detection Kit Real-time RT-PCR ORF1ab and N gene Throat swab, sputum, nasopharyngeal swab 500 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400063) 1/28/2020, CE 5 Sansure Biotech Inc. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence probing) Real-time RT-PCR ORF1ab and N gene Throat swab and Bronchoalveolar Lavage Fluid (BALF) samples 200 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400064) 1/28/2020, CE, FDA EUA 6 Shanghai BioGerm Medical Biotechnology Co., Ltd. Novel Coronavirus (2019-nCoV) Nucleic Acid Detection Kit Real-time RT-PCR ORF1ab and N gene Oropharyngeal, nasopharyngeal, and sputum 500 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400065) 1/31/2020 7 Beijing Applied Biological Technologies Co., Ltd. Multiple Real-time PCR kit for Detection of 2019-nCoV Real-time RT-PCR ORF1ab, E and N gene Sputum and throat swab 200 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400179) 2/27/2020, CE 8 Maccura Biotechnology Co., Ltd. SARS-CoV-2 Fluorescent PCR Kit (for the COVID-19 Coronavirus) Real-time RT-PCR ORF1ab, E and N gene Throat swab and Sputum 1000 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400184) 3/1/2020 9 Wuhan EasyDiagnosis Biomedicine Co. Ltd COVID-19 (SARS-CoV-2) Nucleic Acid test Kit Real-time RT-PCR ORF1ab and N gene Oropharyngeal, nasopharyngeal, and sputum 137 copies/mL Results in ~ 3 h after extraction Depended on the real-time instrument China NMPA (20203400212) 3/12/2020, CE, TGA, Brazil, South Africa, South East Asia 10 Shanghai Fosun Long March Medical Science Co., Ltd. Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit Real-time RT-PCR ORF1ab, E and N gene Throat swab and Sputum - Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400299) 3/24/2020 11 Beijng Kinghawk Pharmaceutical Co., Ltd. Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real time RT-PCR ORF1ab and N gene Throat swab and Sputum 500 copies/mL Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400322) 4/3/2020 12 Jiangsu Bioperfectus Technologies Co., Ltd COVID-19 Coronavirus Real Time PCR Kit Real time RT-PCR ORF1ab and N gene Oropharyngeal, nasopharyngeal, and sputum - Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400384) 4/16/2020 13 Zhejiang Oriental genetic biological products Co., Ltd Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real time RT-PCR ORF1ab and N gene Throat swab and Sputum 500 copies/mL Results in 50-75 min Depended on the real-time instrument China NMPA (20203400520) 5/21/2020, PEUA 14 Shenzhen United Medical Science and Technology Co., Ltd. Real Time PCR Kit for Novel Coronavirus 2019-nCoV (ORF1ab, N) Real time RT-PCR ORF1ab gene Throat swab and Sputum 200 copies/mL Results in ~ 1.5 h Depended on the real-time instrument China NMPA (20203400535) 6/5/2020 15 Beijing NaGene Diagnosis Reagent Co., Ltd Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-fluorescence probing) Real time RT-PCR ORF1ab and N gene Oropharyngeal swab and sputum specimens - Results in ~ 2 h after extraction Depended on the real-time instrument China NMPA (20203400537) 6/9/2020 16 Coyote Bioscience Co., Ltd. DirectDetect™COVID-19 Detection Kit Real time RT-PCR ORF1ab and N gene Oropharyngeal swab and sputum specimens 400 copies/mL Results in 30 min ~ 90 min with the instrument of Mini8 plus 4 samples/run China NMPA (20203400644) 7/13/2020, CE, Mexico, Columbia, Indonesia, the Philippines, Aaudi Arabia, Australia 17 Daan Gene Co., Ltd. of Sun Yat-Sen University Detection Kit for SARS-CoV-2 RNA (Fast PCR-Fluorescence Probing) Real time RT-PCR ORF1ab and N gene Throat swab or sputum 500 copies/mL Results in 50-62 min, with the instrument of AGS4800 8 samples/run China NMPA (20203400749) 9/21/2020, CE 18 BGI Biotechnology (Wuhan) CO., LTD Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (Combined probe anchored polymerization sequencing) Sequencing SARS-CoV-2 Throat swab and Bronchoalveolar Lavage Fluid (BALF) samples 100 copies/mL Gene sequencer DNBSEQ-T7, and Automatic sample loading instrument MGIDL-T7 - China NMPA (20203400059) 1/16/2020/CE Marked/FDA Authorized/PMDA Approved 19 Chengdu CapitalBio Jingxin Biotechnology Co., Ltd. Nucleic Acid Detection Kits for Six Kinds of Respiratory Virus (isothermal amplification based on chip) Isothermal Amplification based on Disk Chip SARS-CoV-2 S and N gene, Influenza A, new Influenza A H1N1 Virus (2009) Influenza A H3N2, Influenza B, RSV Throat swab - - - China NMPA (20203400178) 2/22/2020 20 Ustar Biotechnologies (Hangzhou), Ltd. EasyNAT Diagnostic Kit for 2019-nCoV RNA (Isothermal Amplification Real Time Florescence Assasy) Isothermal Amplification-rt-PCR ORF1ab and N gene Throat swab and sputum specimens 1000 copies/mL Results in 79 min 2 samples/run China NMPA (20203400241) 3/16/2020, CE 21 Anbio (Xiamen) Biotechnology Co., Ltd Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (RNA Hybrid Capture-Immunofluorescence Assay) Hybrid capture-immunofluorescence assay ORF1ab, N and E gene Throat swab and sputum specimens 500 copies/mL Results in 45 min 120 samples in 1 h, instantaneous measurement China NMPA (20203400298) 3/24/2020 22 Rendu (Shanghai) Biotechnology Co., Ltd Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (RNA Hybrid Capture-Immunofluorescence Assay) Magnetic based target RNA capturing technology, Isothermal Amplification and real-time Immunofluorescence assay ORF1ab gene Oropharyngeal swab and sputum 250 copies/mL Results in 90 min 80 samples/run China NMPA (20203400300) 3/26/2020 23 Wuhan Zhongzhi Biotechnologies Inc. Novel Coronavirus 2019-nCoV Nucleic Acid Detection Kit (RNA Amplification Lateral Flow Assay) Isothermal Amplification and gold probe-based Chromatography ORF1ab and E gene Throat swab, sputum specimens, nasopharyngeal swab and Bronchoalveolar Lavage Fluid (BALF) samples 1000 copies/mL Results in 1 h 1 sample/run, continuously detected China NMPA (20203400301) 3/31/2020, CE 24 Wuhan Zhongzhi Biotechnologies Inc. Novel Coronavirus 2019-nCoV Nucleic Acid Detection Kit (Dual amplification) Isothermal Amplification based on reverse amplification and T7 RNA polymerase, and chemiluminescence ORF1ab and E gene Throat swab, sputum specimens, nasopharyngeal swab and Bronchoalveolar Lavage Fluid (BALF) samples 100 copies/mL Results in ~ 3 h Highthroughput, 192 samples in 1 run in 3 h China NMPA (20203400302) 3/31/2020 Note: "-" means information was not available Table 1. Molecular diagnostic tests used to detect viral genetic material in SARS-CoV-2 approved by the NMPA.
-
No. Manufacture, organization name Test name Test type Ab type Sample source Test result time/additional information Country of approval 1 Guangzhou Wondfo Biotech CO., Ltd. Wondfo SARS-CoV-2 Antibody Test (Lateral Flow Method) LFIA based on gold particle IgM/IgG Serum, plasm, and blood Results in 15 min China NMPA (20203400176) 2/22/2020 2 Innovita (Tangshan) Biological Technology Co., Ltd COVID-19 IgM/IgG Antibody Test Kit (colloidal gold method) LFIA based on gold particle IgM/IgG Serum and plasm Results in 15 min China NMPA (20203400177) 2/22/2020 3 Guangdong Hexin Health Technology Co., Ltd COVID-19 IgM Antibody Test Kit (colloidal gold method) LFIA based on gold particle IgM Serum and plasm Results in 15 min China NMPA (20203400199) 3/11/2020 4 Vazyme (Nanjing) Biotech Co., Ltd 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold-Based) LFIA based on gold particle IgM/IgG Serum and plasm Results in 10 min China NMPA (20203400239) 3/13/2020 5 Zhuhai Livzon Diagnostics Inc The Diagnostic Kit for IgM/IgG Antibody to Coronavirus (SARS-CoV-2) (Lateral Flow) LFIA based on gold particle IgM/IgG Serum, plasm, and blood Results in 15 min China NMPA (20203400240) 3/14/2020, CE mark 6 Shanghai Outdo Biotech Co., Ltd. Novel coronavirus (SARS-CoV-2) antibody (IgM/IgG) test LFIA based on gold particle IgM/IgG Serum, plasm, and blood - China NMPA (20203400367) 4/10/2020 7 Beijing Zinxing Sihuan Biotech Co., Ltd 2019-nCoV antibody IgM test (Colloidal Gold-Based) LFIA based on gold particle IgM Serum and plasm Results in 10-15 min China NMPA (20203400457) 5/8/2020 8 Bioscience (Chongqing) Diagnostic Technology Co., Ltd Diagnostic kit for novel coronavirus (2019-nCoV) IgM antibody (Magnetic particle CLIA) CLIA based on magnetic particle IgM Serum Chemiluminescence immunoassay Axceed 260 China NMPA (20203400182) 2/29/2020 9 Bioscience (Chongqing) Diagnostic Technology Co., Ltd Diagnostic kit for novel coronavirus (2019-nCoV) IgG antibody (Magnetic particle CLIA) CLIA based on magnetic particle IgG Serum Chemiluminescence immunoassay Axceed 260 China NMPA (20203400183) 2/29/2020 10 Xiamen InnodxBiotech Co. Ltd. Diagnostic kit for novel coronavirus (2019-nCoV) IgM/IgG antibody (Magnetic particle CLIA) CLIA based on magnetic particle IgM/IgG Serum and plasm - China NMPA (20203400198) 3/6/2020 11 Dynamiker Biotechnology (Tianjin) Co., Ltd. Diagnostic kit for novel coronavirus (2019-nCoV) IgG antibody (Magnetic particle CLIA) CLIA based on magnetic particle IgG Serum and plasm - China NMPA (20203400365) 4/10/2020 12 Dynamiker Biotechnology (Tianjin) Co., Ltd. Diagnostic kit for novel coronavirus (2019-nCoV) IgM antibody (Magnetic particle CLIA) CLIA based on magnetic particle IgM Serum and plasm - China NMPA (20203400366) 4/10/2020 13 Zhengzhou Autobio Diagnostics Co., Ltd 2019-nCoV IgM CLIA chemiluminescence CLIA based on magnetic particle IgM Serum and plasm AutoLumo A2000Plus China NMPA (20203400494) 5/15/2020 14 Zhengzhou Autobio Diagnostics Co., Ltd 2019-nCoV IgG CLIA chemiluminescence CLIA based on magnetic particle IgG Serum and plasm AutoLumo A2000Plus China NMPA (20203400495) 5/15/2020 15 Maccura Biotechnology Co., Ltd. SARS-CoV-2 IgG (CLIA) CLIA IgG Serum and plasm 20 min, sensitivity: 96.24%; specificity: 98.13%; total coincidence rate: 97.15% China NMPA (20203400496) 5/18/2020 16 Maccura Biotechnology Co., Ltd. SARS-CoV-2 IgM (CLIA) CLIA IgM Serum and plasm 20 min, sensitivity: 86.99%; specificity: 100.00%; total coincidence rate:93.25% China NMPA (20203400497) 5/18/2020 17 Bioscience (Tianjin) Diagnostic Technology Co., Ltd Diagnostic kit for novel coronavirus (2019-nCoV) IgG antibody (CLIA) CLIA IgG Serum Chemiluminescence immunoassay Axceed 260 China NMPA (20203400498) 5/19/2020 18 Bioscience (Tianjin) Diagnostic Technology Co., Ltd Diagnostic kit for novel coronavirus (2019-nCoV) IgM antibody (CLIA) CLIA IgM Serum Chemiluminescence immunoassay Axceed 260 China NMPA (20203400499) 5/19/2020 19 Beijing Hotgen Biotech Co., Ltd. Novel Coronavirus 2019-nCoV Antibody Test (Up-converting Phosphor Immunochromatographic Technology) Up-converting Phosphor Immunochromatographic Technology IgM/IgG Serum and plasm Results in 15-20 min, hand-held UPT, UPT-3A-1200, UPT-3A-1800 China NMPA (20203400523) 5/25/2020 20 Beijng Kinghawk Pharmaceutical Co., Ltd. Novel Coronavirus 2019-nCoV Antibody Test (Quantum dot fluorescence immunochromatography) Quantum dot fluorescence immunochromatography IgM/IgG Serum, plasm, and blood Results in 15-20 min China NMPA (20203400536) 6/9/2020 21 BGI Biotechnology (Beijing) CO., LTD Novel Coronavirus 2019-nCoV Antibody Test (ELISA) ELISA IgM/IgG Serum and plasm Results in 2 h China NMPA (20203400567) 6/17/2020 22 Shenzhen YHLO Biotech Co., Ltd. iFLASH-SARS-CoV-2-IgM CLIA IgM Serum and plasm 300 tests/h with iFLASH-3000 China NMPA (20203400769) 9/27/2020 23 Shenzhen YHLO Biotech Co., Ltd. iFLASH-SARS-CoV-2-IgG CLIA IgG Serum and plasm 300 tests/h with iFLASH-3000 China NMPA (20203400770) 9/27/2020 24 Xiamen Aode Biotechnology Co., Ltd. Novel Coronavirus 2019-nCoV Antibody Test (Rare Earth Materials-based Nanofluorescence immunochromatography) Rare Earth Materials-based Nanofluorescence immunochromatography IgM/IgG Serum Results in 15 min China NMPA (20203400776) 9/29/2020 25 Beijing Zinxing Sihuan Biotech Co., Ltd Novel Coronavirus 2019-nCoV Antibody Test (colloidal gold method) LFIA based on gold particle IgG Serum and plasm Results in 15 min China NMPA (20203400796) 10/12/2020 Note: "-" means information was not available Table 2. Serological tests used to detect antibodies to SARS-CoV-2 approved by NMPA.
-
No. Nucleic acid IgM IgG Interpretation Treatment measures 1 + – – Patients may be during the "window period" of SARS-CoV-2 infection, typically within 2 weeks after infection Isolation, observation or clinical treatment 2 + + – May be at early infection phage of SARS-CoV-2 3 + – + May be during the mid and late infection stage or recurrent infection. When the IgG antibody in the recovery period increases by 4 times or more compared with the acute phase, a recurrent infection can be diagnosed 4 + + + The patient is in the active infection phase, a certain immunity to SARS-CoV-2 has already been developed 5 – + – One is likely to be in the acute phase of SARS-CoV-2 infection. Nucleic acid testing results should be confirmed first. Other factors such as rheumatoid factors have been found to cause weak IgM positive or positive tests. The result may suggest that one might have been vaccinated recently Vaccination should be ruled out firstly. Observe, exclude the possibility of false negative of nucleic acid, detect the nucleic acids in different kind of samples once more every 3-5 days, and recheck the antibody level about 7-14 days later to confirm whether elevation appeared. One with both IgM and IgG positive could be diagnosed as a patient. Someone would be isolated according to clinical manifestation and epidemiological history 6 – – + One might have recovered, and the virus has been cleared. The IgG could be detected for a long time in the blood. The result may suggest that one might have been vaccinated previously 7 – ± – One experience the first infection, during an early stage. Thus, the viral load is lower than the lower limit of nucleic acid detection. A small amount of IgM has been produced while IgG has not; a false positive result might be caused by rheumatoid factor. The result may suggest that one might have been vaccinated recently 8 – + + One might be recently infected with SARS-CoV-2 and is during the recovery period. The virus has been cleared, but the IgM has not been reduced to the lower limit of detection; or the nucleic acid test result might be false negative and the patient is indeed in the active infection stage. The result may suggest that one might have been vaccinated recently Note: "+", positive; "-", negative; "±", weak positive Table 3. Interpretation of the clinical status of individuals based on nucleic acid and antibody detection results.
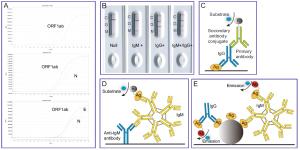
Figure 2 个
Table 3 个