-
Mammary carcinomas are currently the most common and malignant tumor occurring in women. About 1 200 000 women in the world are diagnosed with mammary carcinomas each year and 500 000 women die from this kind of tumor; thus it presents a major threat to women's health and to the quality of their lives. Much attention has been focused on understanding the mechanism of breast carcinogenesis and research has revealed that there are multiple factors and steps involved in this process. In general, in the transformation from normal cells into cancerous ones, many types of DNA damage induce the inactivation, loss or variation of some oncogenes and anti-oncogenes. Such DNA damage may be caused by heredity effects, environmental carcinogens, radioactivity, and viruses. Out of all of these different mechanisms, the relationship between virus and tumor has been the subject of many recent studies. However, although some research have shown that the infection of HPV is closely related to the carcinogenesis of human breast, the precise mechanism is still not clear.
Additionally, research has indicated that HPV-16E6 & E7 can induce cells to be immortal and transform them into tumor cells by influencing the P53 and RB1 proteins, cell cycle regulatory proteins, as well as telomerase.Whether the infection of HPV can induce mammary carcinogenesis via other routes hasn't been reported yet.
In this study, the expression of the HPV-16, iNOS, P53 and hTERT proteins were investigated to examine their possible involvement in the mechanism of HPV in mammary carcinogenesis. If it was found these proteins played a significant role in this process, this may provide a new way to investigate the mechanism in mammary carcinogenesis and provide new options for the clinical prevention of this disease.
HTML
-
52 samples of mammary carcinomas and 16 samples of benign breast tumors from four hospitals in Harbin were assayed. These hospitals were the Thoracic Hospital of Harbin, the 4th hospital of Harbin, the 4th hospital of Harbin Medical University and the 7th hospital of Harbin.
The patients were all female with ages ranging from 23 to 72. The average age was 51. A summary of the types of breast carcinoma in each patient is summarized in Table 1.

Table 1. Types of 52 cases of breast carcinoma
-
Immunohistochemical staining was performed with the two-step method. The sections were deparaffinized and incubated with 3% hydrogen peroxide to in-activate endogenous peroxides, antigen retroviral treatment involved 10 mmol citric acid buffer in a autoclave. Monoclonal antibody HPV-16 (MAB-0354 -working dilution 1:100 was purchased from Boster Biological Technology Ltd), Monoclonal antibody P53 (MAB-0142 -working dilution 1:100) and its auxiliary S-P reagent was purchased from Maixin Biological Technology Ltd. Rabbit Anti-NOS2(BA-0362-working dilution 1:100) and Rabbit anti-Htert (BA-0564 -working dilution 1:100) and its auxiliary S-P reagent was purchased from Boster Biological Technology Ltd. Reaction products were visualized with diaminobenzidine as the chromogen and finally counterstained with hematoxylin.
Following the manufacturers' instructions, we used the given positive section to be the positive control and used PBS instead of the first antibody as the negative control.
-
The positive expression of HPV-16, iNOS and hTERT was determined by the presence of brown granules in the cytoplasm. The positive expression of P53 was determined by the presence of brown granules in nucleus. The criterion for positive expression was that more than 10% of the cells were positive in a sample set of 500 cells.
-
The t-test was adopted to analyze the difference of the expression of the HPV-16, iNOS, P53 and hTERT proteins between the group of 52 samples of breast cancer and the group of 16 samples of benign breast tumors. The x2 test was adopted to show the effect of HPV infection on the expression of NOS, p53 and hTERT proteins and the relationship between HPV, iNOS, P53 and hTERT expression. The Bonferroni correction was used to compensate for the multiple x2 tests.
Clinical materials
Reagents and methods
Analysis of results
Statistical analysis
-
In this study of the expression of HPV in a study group of 52 breast cancer subjects, 23 cases were found to express HPV-16 whereas only one case of HPV infection was found in the benign tumours, corresponding to positive rates of 44.2% and 6.3% respectively. This differerence was found to be significant at P < 0.05 (Table 2). For iNOS, the positive rates were 57.7% and 12.5% respectively (significant at P < 0.05. Table 3). For P53, positive rates were 59.6% and 0% (significant at P < 0.05. Table 4) and for hTERT, positive rates were 65.3% and 18.8%, (significant at P < 0.05, Table 5).

Table 2. The Expression of HPV16

Table 3. The expression of iNOS

Table 4. The expression of P53

Table 5. The expression of hTERT
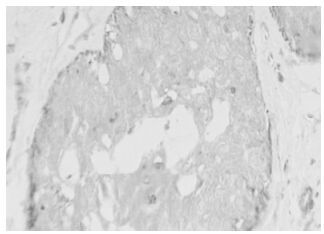
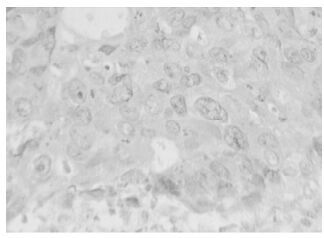
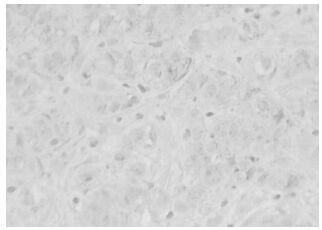
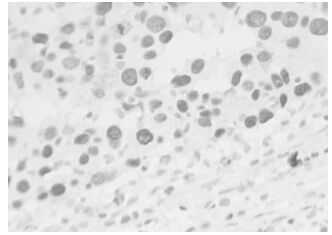
The positive expression of HPV16 in invasive ductal carcinoma of breast with brown granules in the cytoplasm of cancer cells and its estimated percentage was 90% as in shown in Fig. 1. The positive expression of iNOS in papillar carcinoma of breast is shown in Fig 2. There were brown granules in the cytoplasm of cancer cells and the estimated percentage of positive expression cells was 95%. Fig. 3 shows the positive expression of hTERT in invasive ductal carcinoma of breast. There were brown granules in the cytoplasm of cancer cells and the estimated percentage was 90%. The positive expression of P53 in scirrhous carcinoma of breast is shown in Fig. 4. There were brown granules in the nucleus of cancer cells and the estimated percentage of positive expression cells was 85%.
-
The samples were then grouped according to whether or not they had an HPV-16 infection. The positive expression of iNOS was observed in 78.3% of the samples in the HPV-16 positive group and in 41.4% of the samples in the HPV-16 negative group. This difference was significant at P < 0.05. The positive expression of P53 was observed in 87% of the samples in the HPV-16 positive group and 37.9% of the samples in the HPV-16 negative group which was significant at P < 0.05. The positive expression of hTERT was observed in 87% of the samples in the HPV-16 positive group and in 44.8% of the samples in the HPV-16 negative group which was significant at P < 0.05 (Table 6).

Table 6. Affection of Infection of HPV16 on Expression of iNOS, P53 and hTERT in Breast Cancer
-
The positive expression of iNOS was observed in 78.3% of the HPV 16 positive group. The relationship between the expression of HPV16 and the expression of iNOS was found to be significant at P < 0.05 (Table 7). But when the Bonferroni correction was applied (P=0.0167), the relationship between them was not certain. Positive expression of P53 was observed in 76.7%(23/30) of the iNOS positive group and 27/31 cases expressed both hTERT and P53. The expression of iNOS was related to the expression of P53(with Bonferroni correction P=0.0167), as shown in Table 8. The positive expression of hTERT was observed in 87.1%(27/31) of the P53 positive group. The expression of P53 was related to the expression of hTERT(with Bonferroni correction P=0.0167), as shown in Table 9.

Table 7. The Correlation of the Infection of HPV16 with the Expression of iNOS in Breast Cancer

Table 8. The Correlation of the Expression of iNOS with the Expression of P53 in Breast Cancer

Table 9. The Correlation of the Expression of P53 with the Expression of hTERT in Breast Cancer
The expression of HPV-16, iNOS, P53 and hTERT
Effect of HPV infection on expression of NOS, p53 and hTERT
The relationship of HPV, iNOS, P53 and hTERT
-
HPV has been identified as playing an important role in many cancers, including cervical carcinomas, but has also been linked to mammary carcinogenesis. However the mechanism of HPV infection in carcinogenesis is still unclear. Some researchers have suggested that proteins HPV-16E6 and E7 induce cells to be eternal and turn them into tumorous ones by exerting effects on the P53 protein, the RB1 protein, cell cycle regulatory proteins and telomerase.
The transforming properties of HPV 16 are mediated by the E6 protein that disrupts p53 expression and the E7 protein that interferes with the retinoblastoma tumor suppressor protein. James F, et al reported that E6/E7 expression in the mouse epidermis was correlated with increased levels of the p53, p21, p27, cdk2, cdk4, cdk6, cyclin D1 and cyclin E regulatory proteins (9). The HPV E6 protein can heterodimerize with E6AP and activate transcription of hTERT and E6 proteins which bind to the hTERT promoter at E boxes as a transcriptional activator with E6AP and c-Myc (11).
Nitric oxide (NO), a diatomic radical, is an important factor in carcinogenesis. It has diverse physiological and pathophysiological roles as a vasodilator, neurotransmitter, antimicrobial effector molecule, and immunomodulator (8). NO is synthesized from the amino acid L-arginine by the NOS As a free radical, NO is highly reactive and reacts in biological systems with other free radicals, molecular oxygen, and heavy metals and the biological effects of NO are mainly mediated by the products of different NO metabolites (1).
There are three isoforms of NOS: iNOS (NOS2), eNOS (NOS3), and nNOS. eNOS and nNOS are constitutive calmodulin-dependent enzymes. iNOS is expressed in macrophages, neutrophils, endothelial cells, hepatocytes, cardiac myocytes, chondrocytes, and many other cell types (10). iNOS is expressed in a variety of human cancers (2, 4, 7, 16). It is induced most importantly by cytokines and can generate locally high concentrations of NO for prolonged periods of time (14). A large number of cytokines can be produced in inflammation, such as TNF, IL-1 and IFN-γ. Chronic inflammation has been considered to be a factor in carcinogenesis.
The genotoxicity of NO is attributable to its reaction with either oxygen or superoxide (6). NO causes DNA damage by nitrosative deamination, DNA strand breakage or DNA modification (e.g., nitration) by peroxynitrite. The iNOS activity is closely associated with the p53 mutation (3). Liu L reported that mutant p53, iNOS may interact with each other and form the positive and negative feedback loop in ovary carcinogenesis and promote its progression (12).
The expression of iNOS in breast carcinoma has not been studied in such detail, but iNOS is generally present in breast carcinomas and plays an important role in the metastasis and prognosis of breast carcinomas (13). The positive expression of iNOS was observed in 67.5 % of mammary carcinomas, but wasn't observed in normal tissues (15). The positive expression of iNOS was observed in 78% of mammary infiltrating ductal carcinomas and 75% benign tumors, but the intensity and quantity of iNOS in the carcinoma was greater compared to the benign tumor (5).
Our study indicated that although the relationship between them was not certain because of insufficient specimens, there were some indication that the positive expression of HPV 16 may be related to the expression of iNOS. In our study, iNOS was related in turn to the expression of mutated P53 and hTERT. Thus HPV might play an important role in mammary carcinogenesis. The mechanism of HPV infection in mammary carcinogenesis may be as follows:
When the breast is infected with HPV, chronic inflammation takes place. A large number of cytokines can be produced, such as TNF, IL-1 and IFN-γ. This results in the expression of iNOS. The NO produced by iNOS induces the mutation of wild type p53 and the mutation of wild type p53 in turn promotes the expression of hTERT. The up-regulation of hTERT may activate telomerase which stabilizes the length of its telomeric DNA, and so the cell becomes immortalized. Thus this chain of events may ultimately induce mammary carcinogenesis.













 DownLoad:
DownLoad: