-
Cellular immune response such as T cell production of IFN-γ is taken as a measure of vaccine immuno-genicity in experimental vaccine trials, especially for new vaccines such as those against tuberculosis and human immunodeficiency virus (HIV). Currently, a variety of methods, including proliferation assays, cytotoxic T lymphocyte assays, tetramer staining, intracellular cytokine staining and cytokine enzyme-linked immunospot (ELISPOT) assays can be used to assess cell-mediated immune responses (1, 7). Efficient clinical trials to evaluate vaccination aimed at eliciting specific T cells require rapid, accurate, and reliable methods for detecting and quantifying these cells. The interferon (IFN)-γ ELISPOT assay is a method for determining the number of individual T cells secreting this cytokine after stimulation with a specific antigen or peptide (8, 10). The number of spots increases in proportion with the strength of the immune response. The important advantages of the IFN-γ ELISPOT assay are that it directly measures T cell-mediated immune responses, is relatively simple to perform, does not require large numbers of cells and is sensitive. Hence, it is widely used for monitoring the effec-tiveness of vaccines for inducing cell-mediated immunity (5). Several groups in China are actively involved in HIV vaccine research with the aim of eliciting both humoral and cellular immune responses. As these investigations move into preclinical and clinical trials, it will be important to objectively assess the qualitative and quantitative differences in the cellular responses elicited by the different vaccine candidates. However, since the ELISPOT is a proliferation assay for the detection of T cells, many factors including the assay format, nature of the cells being analyzed, choice of antigens and method of plate reading may affect the results. In order to assess the competency and comparability of different laboratories when performing ELISPOT assays, a quality control panel is required. Generally, the volume of a quality control panel should be sufficient for supply and its quality should be stable for a certain time period. In the present study, the possibility of using cryopreserved peripheral blood mononuclear cells (PBMCs) as a quality control panel for ELISPOT was preliminarily evaluated.
HTML
-
Blood collection tubes containing ethylenedia-minetetraacetic acid (EDTA) and lithium heparin as anticoagulants, respectively, were purchased from BD Biosciences (BD Biosciences, San Jose, CA) and Jishuichuangge company (Jishuichuangge, Beijing, China).
-
Whole blood samples were collected from 17 healthy donors (20-50 years of age) using the blood collection tubes described above. All samples were negative for anti-HIV, anti-HCV and HBsAg. The study was approved by the Local Ethics Committee. PBMCs were isolated from whole blood using standard Ficoll-gradient centrifugation. Briefly, whole blood samples diluted 1:1 with phosphate-buffered saline (PBS) were layered onto HISTOPAQUE®-1077-1 solution (Sigma, St. Louis, MO) and centrifuged at 400×g for 30 min. After removal of the buff layer, the white blood cells were collected and washed twice with RPMI 1640.
-
PBMCs were suspended at a concentration of 5×106 cells/mL in freezing medium A comprising 60% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT) and 40% RPMI 1640 medium at room temperature, an equal volume of freezing medium B comprising 20% DMSO (Sigma, St. Louis, MO) and 80% FBS was added dropwise, with gentle mixing by shaking the tube. After pipetting 1.0-mL aliquots of the resulting cell suspension into 2.0-mL cryovials, the vials were placed into a pre-chilled (4℃) Cryogenic Controlled-Rate Freezing Container (Nalgene, Rochester, NY) that was then placed into a -80℃ freezer. After 16 h, the samples were transferred to a liquid nitrogen tank for storage until testing.
-
For thawing, the cryovials were placed in a 37℃ water bath. As soon as the samples had completely thawed, they were pipetted into 15-mL tubes con-taining a 2-fold volume of complete RPMI medium (RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 10 mmol/L HEPES buffer (HyClone), 1 mmol/L L-glutamine (Gibco-BRL, Gaithersburg, MD), 100 μg/mL of penicillin, 100 U/mL of strep-tomycin (HyClone) and 0.05 mmol/L β-mercaptoe-thanol (Sigma) at room temperature. After two washes of the cells at room temperature, the cell recovery and viability were determined by staining with Trypan blue (Sigma).
-
The CEF peptide Pool contains 32 MHC class Ⅰ -restricted T-cell epitopes from human Cytomegalo-virus, Epstein-Barr virus and Influenza virus; to stimulate the release of IFN-gamma from CD8+T-cells in individuals with certain highly frequent HLA types; the CEF-Pool is useful as a Peptide-specific positive control in ELISPOT, CTL and Intracellular Cytokine Assays using human PBMCs. (4, 9) The peptide-pool was produced by PANATecs Gmbh bioanalytical products and services(Germany), and operated in accordance with the manual..
-
The wells of 96-well polyvinylidene difluoride (PVDF) membrane-backed microtiter plates (BD Biosciences) were coated with 100 μL of an anti-human recombinant IFN-γ monoclonal antibody (BD Biosciences) at a concentration of 5 μg/mL and left overnight at 4℃. The wells were then washed three times with sterile PBS, blocked by incubation with 200 μL of complete medium at 37℃ under 5% CO2 for 1-3 h and washed once with 100 μL of complete medium. Next, 50 μL of complete medium containing CEF peptide pools (4 μg/mL) (PANATecs Gmbh, Tuebingen, Germany) was added to each experimental well, while 50 μL of complete medium was added to each negative control well. Phytohemagglutinin (PHA) M (Sigma) at 5 μg/mL was incorporated as a positive control. Next, 50 μL of PBMC suspension at 6×106 cells/mL was added to each well and the plates were incubated overnight for 16-20 h at 37℃, 5% CO2 and 95% humidity. After three washes of the plates with water and four washes with PBS containing 0.05% Tween-20 (ELISPOT wash buffer), a biotinylated anti-human recombinant IFN-γ antibody (BD Biosciences) diluted to 1.0 μg/mL with ELISPOT wash buffer added at 100 μL/well. After incubation at room temperature for 2 h, the plates were washed four times with ELISPOT wash buffer. Streptavidin-horseradish peroxidase (BD Bioscience) diluted appropriately in ELISPOT wash buffer was added to the assay plates at 100 μl/well and incubated at room temperature for 1 h. After three washes of the plates with ELISPOT wash buffer and three washes with PBS, 100 μL of AEC substrate (BD Biosciences) was added to each well, and spots were developed for 30-50 min at room temperature. The substrate was emptied from the wells, and the wells were rinsed with water to stop the reaction. The plates were allowed to air-dry, and the spots were enumerated using an Image Analyzer system BIOREADER®-4000 (BIOSYS, Karben, Germany) for automated plate scanning, imaging and spot counting.
Seven laboratories in China participated in the present study, of which six were located in Beijing and one was located in Shanghai. Our laboratory was designated Lab1. Different laboratories used different reagents. Specifically, the reagents used in Lab2-Lab4 were from BD Biosciences, and the same as those used in our laboratory, while those used in Lab5, Lab6 and Lab7 were from U-Cytech biosciences, CM Utrecht, Netherlands) DAKEWEI Company (Sheng-zheng, China) and Mabtech (Stockholm, Sweden), respectively. However, the amount of cells and CEF peptide pool concentrations were the same in all the participating laboratories. In each individual labo-ratory, the test was performed strictly according to the manufacturer's instructions, and each sample was tested in triplicate. The results were analyzed using a BIOSYSREADER (BIOSYS, Karben, Germany) in Lab2 and Lab5, and an Immunospot READER (CTL Company, Cleveland, OH) in Lab1, Lab3, Lab4, Lab6 and Lab7. Each laboratory reported their results individually. All samples were shipped in dry ice and kept frozen when they reached the participating laboratories.
-
A randomized block-design ANOVA was used in each of the studies to compare the recoveries and viabilities for different cryopreservation times and the ELISPOT results. The SPSS software package was utilized for all analyses.
Blood collection tubes
Sample collection and PBMC preparation
Preparation of frozen PBMCs and storage
Recovery and viability
CEF peptide pool
ELISPOT assay for detection of secreted IFN-γ
Statistical analysis
-
Whole blood samples from 10 healthy donors were collected using EDTA-containing and lithium heparin-containing blood collection tubes from BD Biosciences and Jishuichuangge Company, respectively. The impacts of the different anticoagulants on the PBMC viabilities and frequencies of IFN-γ-secreting PBMCs in response to CEF pool stimulation were analyzed. The results revealed that the numbers of harvested cells (F=0.362, P=0.781) and SFC (spot forming cells) of IFN-γ-secreting PBMCs (F=0.36, P=0.78, Fig. 1) did not differ significantly among these different anticoagulant-containing tubes.
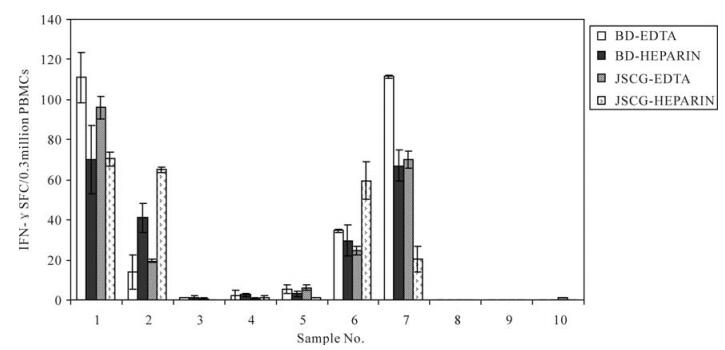
Figure 1. Comparisons of the ELISPOT results for PBMCs isolated from blood collected with EDTA or heparin as the anticoagulant. Data were analyzed by a randomized-block design ANOVA. BD-EDTA, EDTA-containing tube obtained from BD Bioscience; BD-HEPARIN, Heparin-containing tube obtained from BD Bioscience; JSCG-EDTA, EDTA-containing tube obtained from Jishuichuangge Company; JSCG-HEPARIN, Heparin-containing tube obtained from Jishuichuangge Company.
Two fresh PBMC samples were tested by ELISPOT after stimulation with different CEF peptide pool concentrations ranging from 100 to 0.032 μg (five-fold dilutions). The results indicated that the peptide concentration at 0.8-20 μg/mL were significantly more efficient in stimulating the responses.
-
Whole blood samples of approximately 10 mL were collected from 17 healthy donors and the PBMCs were isolated. The PBMCs from each individual were subjected to CEF peptide pool stimulation and tested with ELISPOT. Each sample showed a different performance in the ELISPOT in response to CEF peptide pool stimulation. Overall, 5 samples showed higher responses (SFC/3×105 PBMCs > 200), 8 samples showed medium responses (50 < SFC/3×105 PBMCs≤200) and 4 samples showed lower responses (0 < SFC/3×105 PBMCs≤50). According to the response results, 2 subjects (designated A and D) with higher responses, 2 subjects (designated E and C) with medium responses and 4 subjects (designated B, F, G and H) with lower responses were selected. Whole blood samples of approximately 200 mL were collected from these 8 subjects and the PBMCs were isolated. The isolated PBMCs (5×106 PBMCs/mL) were aliquoted at 1 mL/vial, and stored in liquid nitrogen until further testing. Approximately 40 vials were obtained for each individual.
-
The recoveries and viabilities of PBMCs after different cryopreservation times were analyzed in lab 1. Although the recoveries and viabilities for each sample fluctuated at different cryopreservation times, they were not regularly decreased with extension of the cryopreservation time. The median recoveries for the 8 selected samples at 1, 2, 4, 8, 16 and 24 weeks was 66%, 57%, 70%, 51%, 62% and 65% respectively, producing an average recovery over all the cryopreservation times of 62% with a range of 50-70% (Fig. 2). The median viabilities for the 8 selected samples at 1, 2, 4, 8, 16 and 24 weeks were 98%, 92%, 96%, 92%, 95% and 91%, respectively, producing an average viability over all the cryopreservation times of 94% with a range of 90-100% (Fig. 3). There were no significant differences in the recoveries and viabilities at the different cryopreservation times.
-
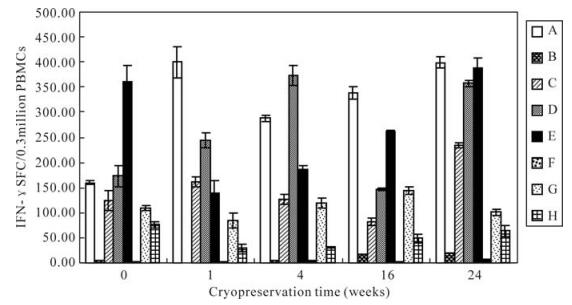
After different cryopreservation times, frozen PBMCs were thawed and subjected to ELISPOT in order to compare their responses with those of freshly isolated (not frozen) PBMCs. As shown in Fig. 4, the SFC was consistent for donors showing both low and high responses. Although the results of ELISPOT fluctuated for the different cryopreservation times, the function of PBMCs frozen for 24 weeks was not significantly decreased compared with that of fresh PBMCs.
-
The spot counts for CEF peptide pool stimulation for each sample are shown in Fig. 5, where samples with high backgrounds, poor replicates or lack of PHA response were excluded. As expected, in samples with no or low IFN-γ responses, there was a wide range of SFC among the laboratories. In samples with medium to high responses, the IFN-γ SFC values were clustered together with a few notable outlying values in some laboratory sample combinations. Despite the presence of the outlying values, there was a clear ability of the seven laboratories to group samples into low, medium and high CEF-responders. These data indicate that, despite the variety of ELISPOT techniques used, this group of laboratories could effectively rank samples according to the strengths of their responses, thereby offering a further level of refinement to the simple determination of the presence or absence of a response. However, when we compared the results obtained in the seven different laboratories, we found that the IFN-γ SFC values for each sample differed significantly among the different laboratories (P=0.019, F=3.454).
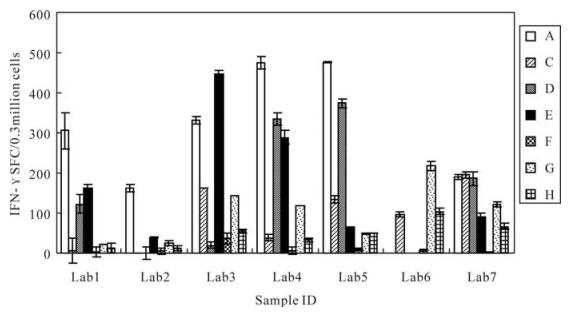
Figure 5. Frequencies of IFN-γ-secreting cells obtained by seven different laboratories. Lab1, Vaccine 1 division of National institute for the control of pharmaceutical and biological products(NICPBP); Lab2, Vaccine 3 division of NICPBP; Lab3, Vaccine 2 division of NICPBP; Lab4, Institute of Virology, Chinese Academy of Preventive Medicine; Lab5, Cytology division of NICPBP; Lab6, The center for disease control and prevention of China; Lab7, The Center for Disease Control and Prevention of Shanghai. A-H represent eight frozen samples respectively.
Comparison of the different blood collection tubes and peptide titration
Selection of cryopreserved PBMCs
Recoveries and viabilities of PBMCs
Comparison of the frequencies of IFN-γ-secreting cells
Frequencies of IFN-γ-secreting cells
-
The ELISPOT tests quantifying antigen-reactive T cells are now increasingly being used to monitor clinical vaccination trials aimed at eliciting specific CTL responses (2). Though ELISPOT assay has the advantages of high sensitivity, direct measurement of ex vivo T cell-mediated immune responses and relatively simple to perform, the reliability of the ELISPOT assay in quantifying T cell frequencies in multisite studies remains to be established. Considering the evaluation of vaccine elicited immunogenicity in large clinical trials generally involve multiple laboratories, it is therefore critical for such clinical trials to be performed according to standardized operating proce-dures (10, 11). Therefore, we investigated the possibi-lity of using cryopreserved PBMCs as a quality control panel for ELISPOT in the present study.
We performed comparative studies for the methods of collection and preservation of samples, since the methods of collection, separation and preservation of PBMCs may differ significantly among individual laboratories. In order to investigate possible sources of variations in the ELISPOT results, we initially examined the use of two different kinds of anticoagulant-containing blood collection tubes to clarify whether different anticoagulants could affect the viability and function of PBMCs. The results showed that the survival abilities of PBMCs and frequencies of IFN-γ-secreting cells after CEF peptide pool stimulation were the same for samples collected in the different anticoagulant-containing tubes purchased from BD Biosciences and a Chinese company. We also investigated the CEF peptide pool concentrations required for stimulation. The results revealed that the IFN-γ SFCs of PBMCs stimulated with various CEF peptide pool concentrations (ranging from 100 to 0.032 μg/mL) were consistent.
The preservation methods used for PBMCs involve the application of cryopreservation fluids to cells and freezing in liquid nitrogen. However, the cell preservation time in liquid nitrogen has not been reported in the literature. Therefore, in order to explore this parameter and its effect on ELISPOT samples, we collected peripheral blood samples from 17 healthy subjects, and isolated the PBMCs for testing by ELISPOT. According to the ELISPOT results, 8 samples with different performance levels in response to CEF peptide pool stimulation were selected to prepare a quality control panel. PBMCs frozen for 1, 2, 4, 8, 16 and 24 weeks were examined for their recoveries, viabilities and ELISPOT results. We found that use of cells after different cryo-preservation times did not affect the viability and function of the panel, even after cryopreservation for 24 weeks. These results indicate that cryopreserved cells can be applied to ELISPOT analysis and that it is possible to develop ELISPOT standard samples for assessing variations among different laboratories. Our results are consistent with those from a previouly published study (7). Our work lays a further foundation for the establishment of an ELISPOT standard panel.
Since the operation procedure of ELISPOT assays is very complex and cumbersome, the ELISPOT results of different laboratories may show great differences. To assess the competency and comparability of laboratories while performing ELISPOT assays, we chose seven different laboratories and organized these laboratories to complete the ELISPOT test according to their respective ELISPOT laboratory operating procedures and conditions, but with the same cell and CEF peptide pool concentrations. The results revealed that, although each laboratory could classify high, medium and low diversities of the responses, there were significant differences in the ELISPOT results among the seven laboratories. It is likely that differences in the experimental operations and detection equipment exist in different laboratories. ELISPOT assays were evaluated between two groups, according to the percent responders and fold differences in the geometric mean. When the results were judged by these criteria (a positive response in the ELISPOT assay was defined as at least 55 SFC/1×106 PBMCs and at least a 4-fold higher value than non-antigen mock control wells), there were no differences among the laboratories. According to previous results for 11 laboratories in the USA, the median recovery and viability before plating for all samples were 35% and 86%, respectively, with notable inter-laboratory and intra-sample variabilities. Remarkable concordance between laboratories was obtained in defining a qualitative assessment of the responder/non-responder status to antigens, but the frequencies of the responding cells varied among the laboratories. This study highlights the need for better standardization of protocols and reagents to obtain reliable and reproducible data that may support immunogenicity studies, vaccine regulatory submissions and licensure (3). Therefore, it is necessary to establish standard operation procedure for ELISPOT.
In conclusion, we have carried out preliminary analyses of the factors for ELISPOT, established standard samples with different reactions to CEF peptide pool stimulation and supplied quality control as a foundation for the ELISPOT assay in the present study. The results show that cryopreserved PBMCs could be used as a quality control panel for ELISPOT. However, the procedures for ELISPOT need to be standardized in different laboratories (6).















 DownLoad:
DownLoad: