-
Herpes simplex virus 1 (HSV-1) infection is common worldwide, with 45% to 98% of the world population and 40% to 63% of the people in the United States reportedly HSV-1 seropositive (7). The prevalence of HSV-1 varies with age, race, geographic location, and socioeconomic status. HSV-1 is trans-mitted via oral secretions and typically infects the squamous surfaces of the lips and mouth. Although HSV-1 infection is principally associated with oral and facial diseases, it can affect any other organs. Such is the case with some genital herpes (6, 16, 46). Most diseases caused by HSV-1 are self-limiting; however, they can be life-threatening in immuno-compromised patients or neonates. Besides, HSV-1 can establish lifelong latent infection in sensory neurons and recurrent lesions at or near the point of entry into the body.
The 152-kb double-stranded DNA genome of HSV-1 comprises 36 genes classified into three temporally regulated groups according to their transcription sequence: Immediate-Early(IE) genes, Early genes and Late genes. More than 70 proteins that act at multiple stages in the viral life cycle are encoded by the genome. Although in theory any of the proteins essential for viral replication is a potential drug target, nearly all clinically effective drugs are nucleoside analogues, which target at the viral DNA polymerase, acting as chain terminators. Following a long period of use of nucleoside analogues, drug resistant virus strains have emerged, especially in patients with AIDS the prevalence of acyclovir (ACV)-resistant strains is remarkable (49). Therefore, the search of novel anti-HSV-1 agents is of great importance.
HTML
-
Medicine and natural products have been closely linked through the use of traditional medicines and natural poisons (10, 34). Today, despite competition from other drug discovery methods, natural products are still providing their fair share of new clinical candidates and drugs. In the total drug launches from 1981 to 2006, of the 155 small molecules, 73% are other than synthetic, with 47% actually being either natural products or directly derived there from in the field of cancer; in other areas, the influence of natural product structures is quite marked, with the anti-infective products being dependent on natural products and their structures (35).
Traditional medicines have been used to prevent or treat HSV-1 infections for a long time. Accordingly, a large number of natural products, including pure compounds and standard extracts, isolated from traditional herbs or other plants, have been examined for their antiviral effects on HSV-1. As an important source of novel anti-HSV-1 agents, natural products deserve further screening, studying and evaluation. In 2004, Khan et al reviewed anti-HSV substances from herbal medicines, reported in studies from several laboratories(19). Based on this, the purpose of this paper is to review the recent status of natural products, especially pure compounds from plants showing anti-HSV-1 activities, reported from 2005 to 2008.
-
Seaweed is an important source of polysaccharides with anti-HSV-1 activities. Several laboratories have isolated different polysaccharides and studied their effects on HSV-1 in vitro or in vivo. Adhikari et al (1) and Mandal et al (31) studied the sulfated fucoidan from brown seaweed Stoechospermum marginatum and Cystoseira indica respectively. Both kinds of fucoidan they isolated showed no cytotoxicity for Vero cell and inhibited virus adsorption; however, they had no direct inactivating effect on virions in a virucidal assay.
A sulfated fucoidan from brown seaweed Undaria pinnatifida was studied for its effects on in vivo viral replication and the host's immune defense system (13). Oral administration of the fucoidan protected mice from infection with HSV-1, as judged from the survival rate and lesion scores. It is suggested that oral intake of this fucoidan might offer protective effects through direct inhibition of viral replication and stimulation of both innate and adaptive immune defense functions.
SP-2a, a sulphated polysaccharide, was isolated from the brown seaweed Sargassum patens (Kütz.) Agardh (Sargassaceae) (48). This polysaccharide significantly inhibited the in vitro replication of both the ACV-sensitive and -resistant strains of HSV-1, in dose-dependent manners, with 50% inhibition occurring with 1.5-5.3 μg/mL of the polysaccharide. Mechanism studies demonstrated that the antiviral mode of action of SP-2a is mediated mainly by inhibiting virus attachment to host cells, and this sulphated polysaccharide might have different modes of action against the ACV-sensitive and -resistant strains of HSV-1.
Polysaccharides showing anti-HSV-1 activities from other algas were also reported. Two sulfated galactans from red seaweed Grateloupia indica and Schizymenia binderi (Gigartinales, Rhodophyta) were studied respectively (4, 32), which showed effective inhibition against HSV-1. The sulfated galactan from S. binderi was shown to interfere with the initial adsorption of viruses to cells. In addition, a partially cyclized μ/v-carrageenan 1C3, isolated from the red seaweed Gigartina skottsbergii, was another potent inhibitor of the in vitro replication of HSV-1 reported by Pujol et al (37). In their study, the protective effect of 1C3 in a murine model of intraperitoneal (i.p.) HSV-1 infection was evaluated and pharmacokinetic properties were analyzed after injection of 1C3 into the tail vein by monitoring of [3H]-1C3 in plasma and organs and by a bioassay of the anti-HSV-1 activity remaining in serum after non-radioactive 1C3 inoculation.
A novel acidic polysaccharide, nostoflan, which may be a potential antiherpetic agent, was isolated from the blue-green alga, Nostoc flagelliforme (17, 18). The target for its anti-HSV-1 action was examined. The results of virus binding and penetration assays indicated that the inhibition of virus binding to but not penetration into host cells was responsible for the antiherpetic effect induced by nostoflan.
Glycoconjugate is another kind of anti-HSV-1 agent. The chemical nature, mode of action, and in vitro and in vivo anti-HSV activities of a lignin-carbohydrate complex (PPS-2b) from Prunella vulgaris were characterized (47). PPS-2b inactivated HSV-1 directly, blocked HSV-1 binding to Vero cells, and inhibited HSV-1 penetration into Vero cells. Li et al obtained a bioactive fraction Ganoderma lucidum proteoglycan(GLPG) from the mycelia of Ganoderma lucidum. GLPG was a proteoglycan with a carbohydrate: protein ratio of 10.4:1 and inhibited cell death in a dose-dependent manner in HSV-infected cells (27). It may inhibit viral replication by interfering with the early events of viral adsorption and entry into target cells.
Most of the polysaccharides reported are sulfated forms. It is supposed that sulphate groups, if present, appeared to be crucial for the anti-herpetic activity of the polymer (4, 31). The effect of partial desulfation and oversulfation of Na-SP isolated from Spirulina platensis on the exhibition of anti-HSV-1 activity were evaluated (23). Anti-HSV-1 activity of partially desulfated derivatives seemed to be dependent on the degree of sulfation. However, naturally occurring Na-SP might contain sufficient sulfates because no clear dependence of anti-HSV-1 activity on degree of sulfation was observed in the case of oversulfated derivatives. There are other reports on the correlations between degree of sulfation and biological activity such as anticoagulant activity (2, 11). It is well documented that biological activities of sulfated polysaccharides are dependent on their degree of sulfation, as well as molecular weight and polysac-charides structure such as linear or branched one.
-
Polyphenols may be classified into different groups as a function of the number of phenol rings that they contain and of the structural elements that bind these rings to one another (30). Different polyphenols, especially flavonoids, phenolic acids, as reported in recent years, showed anti-HSV-1 activities.
Flavonoids, including flavonols, flavones, flavanones, isoflavone, flavanols and other compounds, contribute to a considerable source of anti-HSV-1 compounds. Anti-herpetic assays on 18 flavonoids in five classes were carried out by Lyu et al (29). Epicatechin (EC), epicatechin gallate (ECG), galangin, and kaempferol showed a strong antiviral activity, and catechin (C), epigallocatechin (EGC), epigallocatechin gallate (EGCG), naringenin, chrysin, baicalin, fisetin, myricetin, quercetin, and genistein showed moderate inhibitory effects against HSV-1. Flavanols and flavonols appeared to be more active than flavones. Furthermore, treatment of Vero cells with ECG and galangin (which previously showed strong antiviral activities) before virus adsorption led to a slight enhancement of inhibition as determined by a yield reduction assay, indicating that an intracellular effect may also be in-volved.
The catechins, classified as flavanols, are the major polyphenolic components of fresh tea leaves as well as the soluble matter in green tea. The main catechins present in green tea are EGCG, EGC, and EC. All of these catechins have the structure of flavan-3-ol and possess additional pyrogallol or galloyl groups that contribute to their biological activities (45). The antiherpetic activity and genotoxicity of catechin and some of its derivatives were studied by Savi L A et al (38). All compounds tested showed antiviral activity with selective indices varying from 1.3 to 13. The molecules containing three hydroxyl groups on the B ring caused less DNA damage and showed better antiviral effect than those with two hydroxyls on the same ring, but if there is an additional galloyl group, these results can be altered. The bioavailability and stereochemistry could be related to the antiviral and genotoxic effects detected. Chemical structures of tested compounds are shown in Fig. 1.
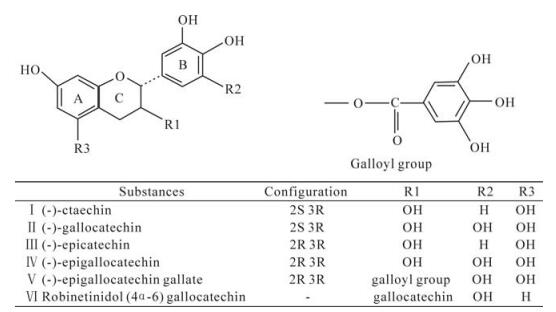
Figure 1. Chemical structures of tested catechin and its derivatives (38).
In another study by Isaacs et al, the anti-HSV-1 activity of EGCG was examined. EGCG has greater anti-HSV-1 activity than other green tea catechins that were tested (15). The anti-HSV-1 activity of EGCG is due to a direct effect on the virion. Besides, electron microscopical (EM) and SDS gel electrophoresis studies demonstrated that EGCG could inactivate HSV-1 virions by binding to gB, gD or another envelope glycoprotein. EGCG is stable in the pH range found vaginally and appears to be a promising candidate for use in a microbicide to reduce HSV transmission.
Moreover, three flavones cycloartocarpin, isocy-clomorusin, norartocarpetin and the stilbene oxyresveratrol from the heartwood of Artocarpus gomezianus were reported to show moderate activities against HSV-1 (24). Flavan-4-ol luteoforol, isolated from Hypericum connatum (Guttiferae), which is used in southern Brazil in the treatment of lesions in the mouth, often related to acute herpetic gingivosto-matitis, shows anti-HSV-1 activity (9). In addition, two new antiviral flavan derivatives, 7-O-galloy-ltricetifavan and 7, 4'-di-O-galloyltricetifavan, isolated from the leaves of Pithecellobium clypearia, possess antiviral activity against HSV-1 (26).
Phenolic acids, another group of polyphenolic compounds, may also possess anti-HSV-1 activities. Uozaki et al examined gallic acid (3, 4, 5-trihy-droxybenzoic acid) and its alkyl esters for their effects on virus growth and virion infectivity. All the compounds tested showed an inhibitory effect on the growth of HSV-1 in HEp-2 or Vero cells (42). It was also observed that octyl gallate can directly inactivate HSV-1 (virucidal activity) and suppresses both the intracellar multiplication and the release of the virus. Furthermore, it selectively accelerates death of the virus-infected cells and the addition of the reagent even at 6-h post infection completely abolishes the formation of progeny virus in the infected cells. Recently, another compound, 1, 3, 4, 6-tetra-O-galloyl-β-D-glucose (1346TOGDG), was isolated from the traditional medicinal plant Phyllanthus urinaria and examined for its activity against HSV-1 in vitro (44). 1346TOGDG effectively inhibited HSV-1 infection, with inhibitory concentration (IC50) at 19.2± 4.0μmol/L and no toxic effect at the antiviral concentration.
As a final example, curcumin (diferuloylmethane), a phenolic compound from the curry spice turmeric (Curcuma longa Linn.), is known to inhibit the histone acetyltransferase activity of the transcriptional coactivator proteins p300 and CBP, which are recruited to the IE gene promoters of HSV-1 by the viral transactivator protein VP16. Kutluay et al ex-amined the mode of action of curcumin in cell culture assays for its anti-herpetic potential (22). Curcumin treatment of HeLa cells at nontoxic levels slows HSV-1 replication and decreases the ability of cells to support infection by HSV-1. However, mechanism studies suggest that curcumin affects VP16-mediated recruitment of RNA polymerase Ⅱ to IE gene promoters by a mechanism independent of p300/CBP histone acetyltransferase activity.
-
Terpenes, mainly including sesquiterpenes, diterpenes, triterpenoids, show anti-HSV-1 potential. The merodi-terpenoids atomaric acid, epitaondiol and the peroxy-lactone of 5'a-desmethyl-5'-acetylatomaric acid isolated from the Brazilian brown alga Stypopodium zonale collected in two localities (Búzios and Marataízes, RJ and ES States) showed strong anti-HSV-1 activity in vitro (39). Krawczyk et al investigated the antiviral, antibacterial and cytotoxic properties of 7 new compounds: derivatives of sesquiterpenes originating from Lactarius mushroom and taxol-N-benzoylpheny-lisoserinates of sesquiterpenoid alcohols (20). All compounds showed a lower cytotoxicity than taxol. It was found that out of 7 investigated compounds 3 exhibited anti-HSV-1 activity, which may constitute a potential source of chemotherapeutics.
Three taxol derivatives, 10-deacetyl-baccatin Ⅲ, methyl (N-benzoyl-(2'R, 3'S)-3'-phenylisoserinate) and N-benzoyl (2'R, 3'S) -3'-phenylisoserine were shown to inhibit HSV-1 replication at non-cytotoxic concen-trations in vitro (21). Selectivity indices were in the range 9.5-46.7. Anti-HSV-1 activity of the compounds may be associated with their influence on mitotic division. One compound, methyl (N-benzoyl-(2'R, 3'S)-3'-phenylisoserinate), inhibited T lymphocyte proliferation. It was demonstrated that modified parts of the taxol molecule possess various types of biological activity in vitro and in vivo.
Another three diterpenes, andrographolide, neoan-drographolide and 14-deoxy-11, 12-didehydroan-drog-rapholide, isolated from Andrographis paniculata showed viricidal activity against HSV-1 without significant cytotoxicity at viricidal concentrations (43). Barbosa et al isolated the rare dolabellane diterpene 10, 18-diacetoxy-8-hydroxy-2, 6-dolabelladiene, the new 10-acetoxy-8, 18-di-hydroxy-2, 6-dolabelladiene from Brazilian brown alga Dictyota pfaffii and obtained 8, 10, 18-trihydroxy-2, 6-dolabelladiene by reduction of 10, 18-diacetoxy-8-hydroxy-2, 6-dolabel-ladiene, all three substances showed strong anti-HSV-1 activity in vitro (3).
Two highly active pure triterpenoids, namely 3α-hydroxylup-20(29)-ene-23, 28-dioic acid and 3-epi-betulinic acid 3-O-sulfate were isolated from the leafstalk extract of the medicinal plant Schefflera heptaphylla (25). The two triterpenoids possessed antiviral activity against HSV-1 with IC50 values of 18.8 and 25μg/mL, respectively.
Furthermore, Ikeda et al examined the anti-HSV-1 activity of 15 oleanan-type triterpenoides including glycyrrhizin and its sapogenol(14). It was documented that the in vivo anti-HSV-1 activity of glycyrrhizin administered orally could be reasonably attributed to its sapogenol glycyrrhetic acid generated by hydroly-sis by intestinal bacteria because glycyrrhetic acid showed 10 times greater anti-HSV-1 activity than glycyrrhizin in the in vitro assay. The obtained structure-anti-HSV-1 activity relationships are sum-marized in Fig. 2. The hydroxylation at C-21 seemed to reduce anti-HSV-1 activity, and the C-29 hydroxy group would eliminate anti-HSV-1 activity. On the other hand, a methoxy carboxy group at C-20 might enhance activity.

Figure 2. Structure anti-HSV-1 activity relationships of oleanane-type triterpenoides (14).
-
Natural derived peptides or proteins may possess anti-HSV-1 activities. Filho et al described the purifi-cation of an antiviral peptide from seeds of Sorghum bicolor L. (8). The 2 kDa peptide strongly inhibited the replication of HSV-1, while the presence of the peptide before HSV-1 infections showed moderate inhibition of virus-induced cytopathic effects (CPE) as compared to during or after infections, with EC50 values of 12.5, 6.25, and 6.25muM, respectively. This indicates that the 2kDa peptide was able not only to inhibit the initiation and the spread of infection, but also had an in vitro prophylactic effect against HSV-1 infection.
A novel antiviral protein GFAHP was purified from an extract of Grifola frondosa fruiting bodies (12). GFAHP had a molecular weight of 29.5 kDa. This protein inhibited HSV-1 replication in vitro with an IC50 value of 4.1μg/mL and a therapeutic index > 29.3. Higher concentrations of GFAHP (125 and 500μg /mL) also significantly reduced the severity of HSV-1 induced blepharitis, neovascularization, and stromal keratitis in a murine model. It was proved that GFAHP directly inactivates HSV-1 while simul-taneously inhibiting HSV-1 penetration into Vero cells.
Polysaccharides
Polyphenols
Terpenes
Peptides or Proteins
-
A large number of extracts from plants have been reported to possess anti-HSV-1 activities. Since the components of most active extracts are not clear, related mechanism study is rare. Recently, several South American plants have been studied for their antiherpetic activities (33). From the crude tested extracts, Ilex paraguariensis(aqueous extract–leaves), Lafoensia pacari (MeOH extract–leaves), Passiflora edulis (aqueous extract–roots), Rubus imperialis (MeOH extract-leaves) and Sloanea guianensis (MeOH extract–leaves) were the most active against both strains of HSV-1, with EC50 values rangeing from 60 to 170 μg/mL, and their SIs were higher than 7.
Another two extracts that deserve mention are from Kenyan medical plant Carissa edulis and marine alga Symphyocladia latiuscula respectively. The aqueous total extracts preparation from the roots of Carissa edulis (Forssk.) Vahl (Apocynaceae) significantly inhibited formation of plaques in Vero E6 cells infected with 100PFU of wild type strains of HSV-1 (7401H HSV-1) or resistant strains of HSV-1 (TK-7401H HSV-1 and APr 7401H HSV-1) by 100% at 50 μg/mL in vitro with minimal cell cytotoxicity (CC50=480 μg/mL) (41). The MeOH extract of Sym-phyocladia latiuscula (Rhodomelaceae) and its fractions exhibited antiviral activities against ACV and phosphonoacetic acid (PAA)-resistant (APr) HSV-1, thymidine kinase (TK-) deficient HSV-1 and wild type HSV-1 in vitro without cytotoxicity (36). Both extracts exhibited significant anti-HSV-1 activity in a murine model using Balb/C mice cutaneously infected with HSV-1, suggesting the potent of these extracts as anti-HSV-1 agents.
It is suggested that different extracts exhibit anti-HSV-1 activities because they contain potential agents. These agents need to be isolated and identified and screened for possible anti-viral activity. Such agents could be developed as anti-HSV-1 agents or provide a template for the synthesis of new anti-HSV-1 agents.
-
Plants supply most of the active ingredients of natural antiviral products. Nevertheless, microor-ganisms, animals and fungi also afford potential active agents which may turn to be innovative medicines (5, 28). Several compounds, such as SP-2a, some fucoidans, galactans and glycoconjugates were demonstrated to interfere with the process of viral attachment and entry by time-of-addition experiments; however, the molecular interaction between the compounds and viral particles are still unclear. In fact, for the majority of the identified compounds, further studies intending to reveal their mechanisms of action are still required. These mechanism investigations may in return promote the discovery of new antiherpetic targets and screening methods and therefore facilitate the development of novel anti-HSV-1 compounds.
Given the structure-activity relationship (SAR) studies, natural derived compounds can act as a basis for antiviral drug development. Some anti-HSV-1 compounds and their derivates have been subjected to SAR studies to clarify molecular properties important to their activities. However, the results, as presented before, were comparatively incomprehensive. In order to design new compounds more reasonably, quan-titative SAR studies may be introduced.
Generally, Vero cell-based cytopathic effects (CPE) reduction and plaque reduction assays have been successfully applied to in vitro anti-HSV-1 evaluation of pure compounds or extracts. 3-(4, 5-Dimethylthia-zol-2-yl) -2, 5-diphenyl tetrazolium bromide (MTT) colorimetric assay is also used (33, 38) but has become relatively uncommon in recent years, which may due to the instability of assay results. In this case, sodium 3'-[1-(phenylamino-carbonyl)-3, 4-tetrazolium] -bis (4-methoxy-6-nitro) benzene sulphonic acid (XTT) method can be a good substitute to overcome the drawback of the MTT assay, for the reduction of XTT yields a water-soluble formazan product and eliminates the need for the media removal and fromazan solubili-zation steps before spectrophotometric analysis which will shorten assay process and enhance the reliability of assay results.
Although traditional cell-based screening systems provide opportunities for discovering anti-HSV-1 compounds, the probability is low and the antiherpetic targets are not identified. High throughput screenings (HTS), especially which target specific viral proteins essential for virus replication such as helicase-primase complex (40), represent more efficient means for discovery of novel drugs with clarified action mechanisms. However, more extensive screening of natural products, combined with SAR studies and chemical synthesis of the active compounds and their derivates are required to establish natural product libraries before HTS can be applied to natural-derived drug discovery programs.













 DownLoad:
DownLoad: