-
In recent years, a large number of new and previously identfied viruses are emerging or re-emerging, such as SARS coronavirus, Avian influenza A virus and Nipah virus (7, 11, 14, 19), which seriously threaten the health of the general population. Rapid identification of these viruses plays a vital role in the diagnosis and control of their corresponding diseases; therefore increasing effort is being placed on the technology for their isolation and identification (3, 4, 5, 9, 16, 19). Immunological techniques, especially the development of monoclonal antibodies have provided powerful tools to detect viral particles (3, 4, 16). Molecular detection of viral DNA and RNA is routinely used to rapidly detect and identify known pathogens (1, 2, 4, 19). Compared to traditional cell culture methods, these molecular methods have the advantages of being rapid and simple to use. However, cell culture is still regarded as "the golden standard" for viral isolation, and is also one of the most convincing methods in virus identifi-cation (4). The discovery by John Enders, Thomas Weller, and Frederick Robbins that poliovirus could propagate in cultured cells in 1949 was regarded as a revolutionary finding, for which these three scientists were awarded the Nobel Prize in physiology and medicine in 1954. Their work stimulated the amplifi-cation of many other viruses in cultured cells and also facilitated the discovery of new viruses and the development of many viral vaccines (4, 9, 11, 14). Traditionally, viral isolation by cell culture normally uses individual cell lines for viral infection. This approach becomes less effective when there is a limited supply of specimen and a number of different cell lines have to be used for isolation of novel or unknown viruses. To this end, we have developed a new isolated co-culture cell system which allows virus isolation in multiple cell lines using a small amount of starting materials.
HTML
-
Vero, VeroE6, Hela, HepG2, HEK293 or BHK cell lines were all maintained in Dulbecco modified Eagle medium (DMEM) (GiBCO, Invitrogen, USA) supple-mented with 10% Fetal Bovine Serum (FBS) (GiBCO, Invitrogen, USA) and were grown in an incubator at 37℃ with 5% CO2 (Thermo, United State). The LLC-MK2 cell line was originally maintained in Roswell Park Memorial Institute (RPMI) (GiBCO, Invitrogen, USA) 1640 medium supplemented with 10% FBS, and witho DMEM+10%FBS in our experi-ments. Among the seven cell lines used in this study, six (Vero, VeroE6, Hela, HepG2, HEK293 and LLC-MK2) could support the replication of adenovirus. The BHK cell line, which could not support the propagation of adenovirus, was used as a control. A strain of type Ⅲ adenovirus was kindly provided by CCGVCC (China Centre General Viruses Culture Collection). Anti-adenovirus human serum was kindly provided by Professor Lin Chen from Guangzhou Institute of Biomedicine and Health.
-
A 35mm TC-Treated culture dish (Corning, United State) was divided into seven areas by marker as shown in Fig. 1A. Each of the area was seeded with 104 cells of one of the above cell lines. After 4 h incubation in incubator with CO2, the media were carefully removed, and the dish was washed twice with phosphate buffered saline (PBS).
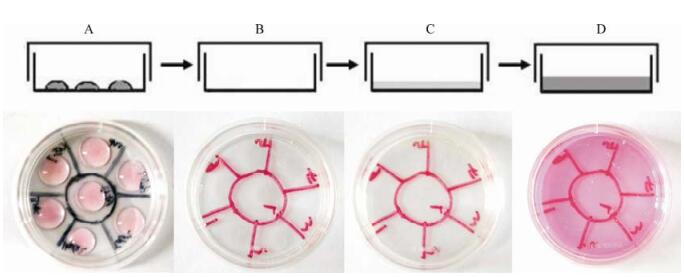
Figure 1. A schematic diagram of the isolated co-culture cell system. A: Seeding of the cells in the seven divided sections; B: After attachment for 4 h, the medium was removed from the dish and unattached cells were washed away with PBS; C: Incubation with a thin overlay of virus solution for 1 h; D: After washing, the maintenance medium was added and the cells were further incubated.
-
Type Ⅲ adenovirus was chosen to test the isolated co-culture cell system because of its wide host-range and low risk. An aliquot of 1.5 mL virus stock was gently loaded to each dish covering the whole surface so that all seven cells received equal exposure of the specimen, and the dish was incubated for one hour at 37℃ in an incubator with CO2. Then the viral medium was discarded, the cells were washed by PBS and 1.5 mL maintenance medium (DMEM+2%FBS) was added. The cells were incubated at 37℃ in a CO2 incubator (Thermo, United States). Cytopathogenic effects (CPE) were observed daily.
-
Adenovirus DNA was detected by PCR. Two oligonucleotide primers were used to amplify the fragment of the hex gene; hex1885 (5'-GCCSCART GGKCWTACATGCACATC-3') and hex1913 (5'-CA GCACSCCICGRATGTCAAA-3') (1). At 48 h post infection (p.i.), 1 μL supernatant of infected cell culture was used as the template for PCR in a 25 μL reaction. Each reaction mixture also contained 2.5 μL of 10×buffer, 1 μL of 10 mmol/L dNTP, 0.5 μL of 20 μmol/L forward and reverse primers, 0.5 μL of 2 U/μL Taq DNA polymerase (BioStar, Canada), and 19 μL of ddH2O. The thermal cycling conditions were 94℃ for 10 min, 30 cycles of 94℃ for 15 s, 55℃ for 30 s, and 72℃ for 30 s, followed by 10 min at 72 ℃. Amplified product was analyzed by electrophoresis in a 1℅ agarose gel and visualized by ethidium bromide staining.
The antigen of the adenovirus was detected by immunofluorescence assay. The medium was dis-carded at 48 h p.i. and cells were washed by PBS. The cells were fixed by incubating in pre-cooled 100% ethanol at room temperature for 10 min and washed with PBS. Then, the cells were incubated with an anti-adenovirus human serum as the primary antibody in 3% BSA for 24 h at 4℃ and then washed twice with PBS. Next, cells were incubated with fluorescein isothiocyanate-conjugated affinipure donkey anti-human IgG (Proteintech Group, United State) in 3% BSA for 60 min at room temperature and then washed with PBS. Cells were observed with a fluorescent microscope (Olympus, Japan).
Cell lines, virus and antibody
Co-culture of cells
Infection with adenovirus
Detection of adenovirus replication
-
As all the cells need to be cultured in the same medium(DMEM+10%FBS), the cells were adapted to DMEM if they were originally cultured in a different medium. This was achieved by increasing DMEM by 10% each time during cell subculture. After one month, Vero, VeroE6, Hela, HepG2, HEK293, BHK and LLC-MK2 all grew well in DMEM+10%FBS.
Seeding of cells was performed sa described in Materials and Methods. After 4 h attachement, most cells stuck to thd bottom of the dish, and unattached ones were washed away. Thus, only cells which were culturde in these settled and isolated areas remained and it was possible to monitor CPE for disstince region.
-
After 48 h p.i., typical CPE of adenovirus infection could be observed in Vero, VeroE6, Hela, HepG2 and HEK293 cells under the light microscope. The infected cells were rounded and swollen. No obvious differences were observed in the infected LLC-MK2 and BHK cells in comparison to the un-infected controls. (Fig. 2)

Figure 2. The isolated co-culture cells infected with adenovirus. At 48 h p.i., CPE was observed in the Vero, VeroE6, Hela, HepG2 and HEK293 cells, but not in the LLC-MK2 and BHK cells.
The supernatant of infected co-cultured cells was used as the template for PCR identification. Using adenovirus specific primers hex1885 and hex1913, a PCR product with a size of 300 bp could be detected (Fig. 3). The expected size of the PCR product of the adenovirus was 301 bp, therefore the PCR result in-dicated that the adenovirus was replicated in the co-cultured cells.
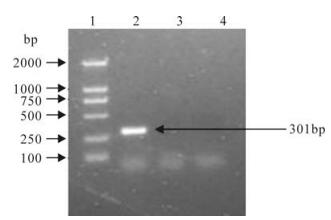
Figure 3. PCR for identification of infected isolated co-culture cell supernatant. Lane 1, DL2000 marker; Lane 2, Infected isolated co-culture cells suspension; Lane 3, Uninfected isolated co-culture cells suspension; Lane 4, Negative control of PCR.
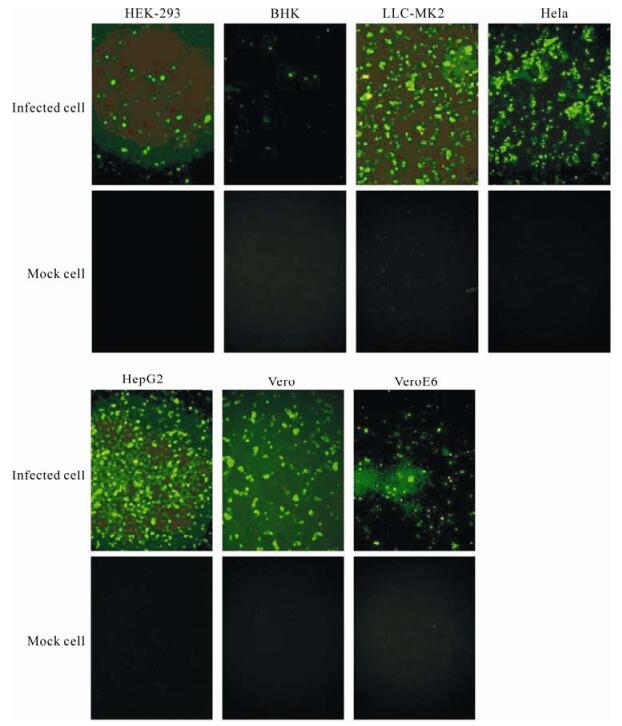
Immunofluorescence assay was used to detect the adenovirus antigens of the co-cultured cells. As shown in Fig. 4, at 48 h p.i., strong fluorescence could be detected in the infected co-cultured HEK-293, Hela, HepG2, LLC-MK2, Vero, and VeroE6 cells, but not in the BHK cells under the fluorescence microscopy. (Fig. 4).
Development of the isolated co-culture cell system
Amplification and detection of adenovirus in the isolated co-culture cells
-
The development of new research fields frequently depends on the development of new techniques. It was in 1907 when Ross Harrison successfully introduced a new technique, tissue culture, which caused revo-lutions in cell biology, virology, immunology, bioen-gineering, medicine and many other areas (8).
Viruses are obligate intracellular parasites. Cultured cells, eggs and laboratory animals are the most common methods for virus isolation. However, although eggs and laboratory animals are very useful for the isolation of certain viruses, cell cultures are the main system for virus isolation. Although molecular methods have become more widely used in the field of virus detection, cell culture remains the golden standard for viral isolation.
Different cell lines vary greatly in their suscepti-bility to different viruses. It is of utmost importance that the most sensitive cell cultures are used for iso-lating a particular suspected virus. When the pathogen is unknown, the traditional approach is to inoculate the specimen into several different types of cell cultures until CPE is observed (6, 10, 12, 13, 15, 17, 18). The success of viral isolation is very much dependent on the experience of the researcher, and when the specimen quantity is limited, the success rate is often low. In this study, an isolated co-cultured cell system that could support several different cell lines to be maintained in the same container was developed. The cells were seeded in isolated zones of the container and were co-cultured with the same medium. It increases the opportunity of the virus to encounter its favoured cells and reduces the use of specimens. Our model infection with adenovirus showed that at 48 h p.i., five permissive cell lines demonstrated typical CPE indicating that the co-cultured cells were suitable for viral detection and isolation. The co-cultured cells may also provide cell factors which could potentially increase the sensitivity of viral infection in otherwise non-susceptible cells, although further investigation is required to provide scientific support for this.
Techniques involving combinations of different cell lines grown together have been applied for detection of several viruses (3, 4). In comparison to the direct mixed-culture method, our system, in which the different cells were cultured in isolation, has several advantages. First, if CPE is detected after infection with an unknown pathogen, the knowledge of which cell line is permissive to the viral infection can aid identification of the virus, minimizing the number offurther experiments required to narrow things down. Second, the CPEs shown in our co-cultured system were similar to the CPEs in the individual cultured cells, while that of the mixed cell culture could be different. Immunofluorescence is widely used for the rapid diagnosis of virus infections by the detection of virus antigen in specimens, as well as the detection of virus-specific antibody (3, 16). It is shown in this study that it can be coupled with the co-culture system for pathogen identification. In fact the immuno-fluorescence assay made the co-culture system more sensitive, as the LLC-MK2 cells which did not show CPE could be detected by immunofluorescence assay.
In summary, in this study an isolated co-culture system was developed which has the potential to be applied torapid isolation of unknown virus when the starting material is limited. Future studies will exa-mine the feasibility of increasing the number of different cell lines in the same dish to further enhance the success rate. Facing with the increasing number of emerging viruses in recent times, it is hoped that this novel virus isolation system will play a important role in rapid isolation and identification of new pathogens in future disease investigation.













 DownLoad:
DownLoad: