-
Human cytomegalovirus (HCMV) is the most common cause of congenital infections in human, with a reported incidence that varies from 0.5%-2.2% (7). Such congenital infections can cause severe birth defects such as microcephaly, mental retardation, motor deficiency, visual impairment and sensorineural deafness (8, 14).
The neuropathogenesis of these brain disorders has not yet been fully understood. HCMV-infected cells are located predominately in the ventricular and subventricular zones (SVZ) (4, 12), where the neural precursor cells(NPCs)are present. Examination of cultures of mouse brain slices prepared at different developmental stages showed that the subventricular zone and cortical marginal regions were most susceptible to murine CMV (MCMV) infection and that this susceptibility declined as the brain developed (4). Our hypothesis is that HCMV infection changes the proliferation, differentiation, or some other biological activity of the human NPCs, and thereby leads to brain abnormalities.
CMV is a species-specific virus, which presents obvious difficulties in investigating its pathogenesis in human. Most of the research in this field has employed murine CMV (MCMV) as a model to study the neuropathogenesis of CMV infection. In vitro, murine NPCs are permissive for MCMV infection, which has been shown to inhibit self-renewal, differentiation, and proliferation (5).
Recent advances in the isolation and culturing of NPCs in vitro provide new possibilities for characterizing the effects of HCMV infection on the functions of these cells. Human NPCs can be expanded in vitro as neurospheres, allowing differentiation into both neuronal and glial phenotypes. In our study, we have observed that human NPCs derived from the Hippocampus were permissive for HCMV AD169 strain infections, and that infection could inhibit differentiation of the NPCs into astrocytes.
HTML
-
HCMV AD169 strain was kindly supplied by Professor Wen-qing Zhang (Department of Microbio-logy, Qingdao University Medical College) and human embryo lung fibroblast (HELF) were used to propagate viral stocks. Infected HELF cultures were harvested at 80%-100% cytopathic effect and subjected to three freeze-thaw cycles. Cellular debris was removed by centrifugation and the supernatant were stored at -86℃. Virus titers were determined using plaque assays on HLF cells.
-
After obtaining written informed consent from pregnant subjects, hippocampus tissue was collected under sterilized conditions from human fetuses aborted between weeks 5-12 of gestation, dissected in Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 (ham) (1: 1) (Invitrogen Corporation) and then subsequently processed for the establishment of neurosphere cultures.
-
The hippocampus tissues samples were dissociated mechanically into single-cell suspensions by Pasteur pipette, centrifuged and washed twice in DMEM/F12 medium. 10mL of cells (105cells/mL) were then cultured in 75-mL non-coated flasks suspended in 1: 1 DMEM/F-12 (Invitrogen Corporation) supplemented with 2 μg/mL heparin (Sigma), 2% B27 (Invitrogen Corporation), 20 ng/mL human epidermal growth factor (EGF, PeproTech Asia) and 20 ng/mL human basic fibroblast growth factor (bFGF, PeproTech Asia), which resulted in the formation of free-floating neurospheres. These cells were maintained at 37℃ in an atmosphere of 5% CO2 with fresh medium added twice each week. The neurospheres were mechani-cally passaged every 7 to 10 d (depending on the rate of growth of the individual culture) using a NeuroCult® Chemical Dissociation Kit in accordance with the manufacturer's instructions (StemCell Technologies Inc) and thereafter recultured in fresh medium as described above. In all cases, the medium used for culturing of neurospheres was free from antibiotics. Herein, we refer to cultured human neural cells grown as free-floating neurospheres as NPCs.
-
The neurospheres (passage 4-8) of human cells obtained as described above were subsequently dissociated (as above) and thereafter suspended in DMEM: F12(1: 1) supplemented with 10% fetal calf serum. The cells were seeded (3×104 cells/well) onto 0.1 mg/mL poly-D-lysine (Sigma, ) coated circular glass cover slips (13 mm in diameter) in 24-well plates.
HCMV AD169 (MOI=5) was added to the culture medium at the time of initiation of differentiation. The cells were then allowed to differentiate for 7 days before fixation and performance of immunocytochemistry.
-
The differentiated or undifferentiated cells on the glass coverslips were first fixed with buffered 4% paraformaldehyde, pH 7.4, for 30 min at room temperature and then permeabilized with 0.1 mol/L PBS containing 0.2% Triton X-100 for 30 min and thereafter preincubated with 5% normal goat serum for 30 min prior to incubation with the primary antibodies. The primary mouse monoclonal antibodies employed were against the following antigens: 1: 100 Nestin (R & D) and 1: 100 immediate-early (IE) protein (Virostat-Inc, USA). The primary rabbit polyclonal antibodies were against the following antigens: 1: 100 Nestin (BOSTER Biological Technology, LTD, China), 1: 100 glial fibrillary acidic protein (GFAP, Beijing Biosynthesis Biotechnology co., LTD, China) and 1: 100 HCMV late protein-pp65 (Beijing Biosynthesis Biotechnology co., LTD, China). These primary antibodies were diluted in 0.1 mol/L PBS containing 0.2% Triton X-100 and incubated with the cells overnight at 4℃. After thorough rinsing in PBS, the slides were incubated with 1: 500 goat-anti mouse IgG conjugated CY3 or with 1: 200 goat-anti rabbit IgG conjugated FITC secondary antibodies. The cell nuclei were stained with DAPI. To ensure antibody specificity, negative control slides were incubated with PBS instead of primary antibody. The numbers and/or percentages of the cells expressing the different antigens analyzed were calculated by counting three fields per glass.
-
15mL Medium and the infected cells were collected after seven days infection. After three times of freeze thawing cycles and clearing of debris by centri-fugation, the product was stored at -86℃ until ready for use. Then 100 μL of this medium was used to infect fibroblasts (MRC-5) and to observe their mor-phological changes 7 days later.
-
Experiments were repeated at least three times and each time the cells belonged to the same hippocampus tissue from the same passage. Statistical significance from the t-test was indicated with P < 0.05 and P < 0.01.
Strains of HCMV
Hippocampus tissue from human fetuses aborted during the first trimester
Establishment of neurosphere cultures from human hippocampus tissue
In vitro differentiation of NPCs and HCMV infection
Immunofluorescence staining and confocal microscopy evaluation
Evaluation of the Virus production by infected cells
Statistical analysis
-
The primary culture of the hippocampus cells were small globular-like with high refraction. Cells began to aggregate and proliferate after 12-24 h of culturing, subsequently forming free-floating neurospheres 48-72 h later. After three passages, numerous neurospheres with distinct borderline and cilia-like microspikes could be seen (Fig. 1A). It was observed that 95%±8% of the cells dissociated mechanically from the neurospheres expressed Nestin, an inter-mediate filament marker of NPCs, and no obvious expression of GFAP, marker of astrocytes (Fig. 1B). This result indicated that the cultured cells were NPCs and not astrocytes.
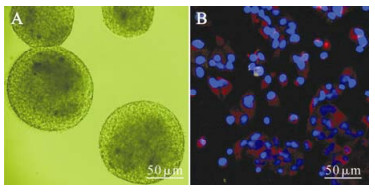
Figure 1. NPCs cultured in vitro developing into free-floating neurospheres with distinct borderline and cilia-like microspikes (A). Immunofluorescence staining show that most of NPCs cultured in this study were Nestin positive (red) and GFAP negative (green). Cellular nuclei were stained by DAPI (blue) (B).
-
Neurospheres cultured in vitro were pipetted up and down into single cells, which were then cultured by D/F12 medium (1: 1) with 10% FBS to differentiate. At the same time HCMV strain AD169 was added to the cells at MOI 5. At 1 to 2 days postinfection (dpi), there were no obvious morphological differences between the infected and control group. Within those groups, the cells turned out to be larger with several irregular spikes and adhered to the cover slip. The infected cells became rounded and swollen at 3 dpi and fused to form giant multinucleated cells at 7 dpi. Immunofluorescence staining showed that the infected cells expressed the HCMV immediate early protein IE and late protein pp65 (Fig. 2). The data showed that HCMV can infect the NPCs. At about 10dpi, the cells detached from the dish and broke into pieces.
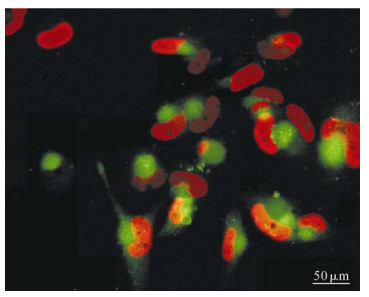
Figure 2. NPCs were infected with HCMV (AD 169) at the onset of differentiation, then cells were fixed at 7d and immunofluorescence staining showed that the infected cells expressing the HCMV immediate early protein IE (red) and late protein pp65 (green).
To assess the production of virus by infected NPCs, the medium of the infected NPCs was collected at 7 days post-infection (dpi), and used to infect fibroblasts, which were fully permissive for HCMV infection. After 3 days of exposure to this medium, the fibroblasts showed clear cytopathic effects (Fig 3). Therefore, these NPCs support complete viral replication, produce and release high levels of mature virus particles.
-
Normal and HCMV-infected NPCs were cultured in DMEM/F12 medium supplied with 10% FBS, which enabled the cells to grow adherently and begin to differentiate into larger and flatter cells with multiple short spikes. With the induction of differentiation, the expression of Nestin in control NPCs decreased, while the expression of GFAP, the marker protein of astrocytes increased. But interestingly, some of the cells appeared both Nestin and GFAP positive, which indicated the dynamic change of these two cell markers. Seven days later, the fluorescence intensity decreased in Nestin positive cells with a proportion of 50% ± 19% (Fig. 4A, Table 1), and the expression of GFAP, the marker protein of astrocytes, increased giving a proportion of 81% ± 11% (Fig. 5A, Table 1). These results suggested that a large percentage of the cells had differentiated into astrocytes. For the HCMV infection group, GFAP-positive cells only represented 55% ± 17% of the total with relatively dim fluorescence expression (Fig. 4B, Table 1) while Nestin was expressed strongly with a high positive proportion of 93% ± 10% (Fig. 5B, Table 1) which suggests that a majority of the infected cells seemed to remain undifferentiated. Together, these findings show that when cultured in DMEM/F12 medium supplied with 10% FBS, most of the NPCs differentiated into astrocytes and HCMV could prevent the differentiation of human NPCs into astrocytes.
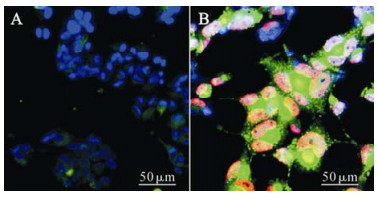
Figure 4. NPCs were infected with HCMV (AD 169) at the onset of differentiation, and 7 days later were fixed and examined for the expression of the HCMV protein IE (red) and Nestin (green) as well as DAPI (blue, nuclear staining). Cells of control group (A) were IE negative while most nuclei of infection group (B) were IE positive. Compared with control group, the positive rate and level of Nestin expression were higher in infected cells.

Table 1. The rate of positive cells in control and infected cells
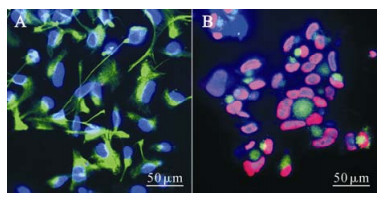
Figure 5. NPCs were infected with HCMV (AD 169) at the onset of differentiation, and 7 days later fixed and examined for the expression of the HCMV protein IE (red) and GFAP (green) as well as DAPI (blue, nuclear staining).Cells of control group (A) were IE negative and GFAP positive while most nuclei of infection group (B) were IE positive. Compared with control group, the positive rate and level of GFAP expression were lower in infected cells.
Neurospheres derived from human fetal hippo-campus were NPCs
NPCs are permissive for HCMV infection
HCMV infection inhibits the differentiation of NPCs into astrocytes
-
NPCs serve as a common source of three major neural cell lineages, which maintain self-renewal and expand to form a pool of undifferentiated cells in the early stage of CNS formation. It is known that the fundamental properties of NPCs are the ability to maintain self-renewal and, later on, undergo stepwise differentiation.
Marker proteins of cells turned out to be an outstanding index for detecting cell differentiation since morphology methods can hardly distinguish differentiated NPCs from undifferentiated ones. At present, the commonly used markers that identify NPCs are Nestin, Vimentin (VIM), Musashi protein and Sox1 protein. Among these, Nestin is the most commonly applied protein marker (2, 3). As a type of intermediate filament protein, Nestin in NSCs begins to be expressed in the neurulation, decreasing in amount in the post-migration stage and shutting off expression at the completion of differentiation. Neurofilament protein, beta-tublin Ⅲ and NSE can be chosen as markers for neurons and GFAP as a marker for astrocytes. In this study, we detected the expression of these marker proteins to determine the proportion of neural stem cells, neurons and astrocytes among these differentiated cells and to evaluate the degree of differentiation in NSCs.
Impairments to the fetus by HCMV infection during pregnancy were mainly localized in the central nervous system (CNS). Epidemiologic studies showed that a higher proportion can cause obvious symptoms such as various kinds of birth defects and neural injuries infected by HCMV mainly within the earlier stages of pregnancy (trimester), and the chances of infection in the late stage are reduced significantly (11). Also, animal experiments have shown that impairment of the CNS in early stage of pregnancy is more serious than those of in middle and late stages (1). The major functional cells in the early stages of pregnancy in CNS are NPCs, from which neurons and glial cells are differentiated. Further research on primates and rodents has shown that HCMV-infected cells are predominately located in the ventricular and subventri-cular zones (SVZ), the regions where the NPCs are present. Therefore, fetal nervous system diseases caused by HCMV infection may be presumed to have close correlation with NPCs damage and abnormalities of proliferation as well as differentiation.
Multiplicities of viral proteins synthesized in the process of HCMV replication cycles will interact with host proteins, which will definitely alter the normal development of host individuals. Nevertheless, according to strict species-specificity and cell tropism, reports studying whether HCMV, which narrowly infects parts of human cells, could infect hNPCs and its pathogenic mechanism (s) are very few. Odeberg first reported that human NPCs coming from the fetal forebrain tissues are susceptible to HCMV infection (TB40 and Towne strain), and this infection inhibits the differentiation of NPCs into neurons and astrocytes, inducing apoptosis in the infected cells (9, 10). In the present study, we have demonstrated that HCMV AD169 strain could establish infection in NPCs derived from the human fetal hippocampus during their differentiation, and cause severe CPE, which ultimately induces death in infected cells. Subsequently, we observed that HCMV infection inhibits the differentiation of NPCs into astrocytes. Normal and HCMV-infected NPCs were cultured in DMEM/F12 medium supplied with 10% FBS, which enables most of the cells to differentiate into astrocytes. However, the expression of Nestin in HCMV-infected cells was still higher after seven days of induction. The proportion of Nestin-positive and GFAP-positive cells were 93% (93% ± 10%) and 55% (55% ± 17%) respectively. It is noteworthy that NPCs tend to retain their undifferentiated conditions following HCMV infection. These data are consistent with the reports of Odeberg (9, 10)and confirm that HCMV infection inhibits the differentiation of human NPCs.
The immediate impairment and arrested differenti-ation will necessarily lead to reduction in cell number and functional defect of NPCs and their differentiated cells (composed of neurons and glial cells). Consequently, this can produce widespread developmental anomalies and impairment such as nervous system malformation, optic nerve atrophy, and mental retardation.
To date, the mechanism of HCMV infection inhibiting the differentiation of NPCs remains unclear but accumulating evidence reveals that during CNS development, multiple signaling pathways, such as WNT, Notch, and TGF-β (6, 13), act as part of a dynamic network to determine the fate of NPCs by modulating the activity of a distinct set of transcription factors which in turn trigger the transcription of neural fate-determination genes. It is highly possible that certain mechanisms may be applied to up-or down-regulate single or multiple signaling molecule(s) after HCMV infection, which ultimately results in differential inhibition. These results show distinct protein expression patterns in NPCs and provide new insight into the pathogenesis of HCMV infection in humans. Future exploration of the mechanisms of HCMV infection inhibiting NPCs will be based on the present study.













 DownLoad:
DownLoad: