-
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), was first identified in 1994 by Chang and colleagues [3]. KSHV has been consistently detected in four clinical epidemiological variants of Kaposi's sarcoma (KS): classic, endemic, iatrogenic and AIDS-associated KS. KSHV has also been detected in primary effusion lymphoma (PEL) and a subset of multicentric Castleman's disease (MCD), particularly in those with HIV infection [2,18].
KSHV is a gamma-2 herpesvirus related to Epstein-Barr virus (EBV). KSHV is a large double-stranded DNA virus of approximately 90 identified open reading frames, and over 60 of them show homology with other rhadinoviruses [20]. KSHV has two replication stages during viral life cycle: latency and lytic replication. During latency, the KSHV does not produce virion particles, and persists as extra-chromosomal episomal DNA circles. Only a few genes are expressed during latency, in KS endothelial cells, the genes of orfK12/kaposin, orfK13/vFLIP, orf72/v-cyclin, and orf73/LANA-1 are expressed; in PEL and MCD B-lymphoma cells, the orfK10.5/LANA-2 is also expressed [19].
The worldwide distribution of KSHV has been extensively investigated using serologic assays measuring antibodies to specific KSHV antigens. The most commonly used serologic assays detect either antibodies to the latency-associated nuclear antigen 1 (LANA-1), to an minor capsid protein encoded by open reading frame (orf) 65 by ELISA or Western blot, or to a virion glycoprotein encoded by orfK8.1 [16]. Using the serologic assays, the seroprevalence of KSHV has been found to be in the order of 40%-60% in sub-Saharan Africa and 20%-40% in South Africa. In the United States, Asia, and Western Europe, the seroprevalence of KSHV is lower than 10%. However, in Mediterranean countries such as Italy, Greece, and Spain, prevalence rates are higher.
KSHV orf73 encodes a latency-associated nuclear antigen (LANA), named LANA-1, which is the basis for several serologic assays [13]. The KSHV orf73 encoded protein is expressed during a latent infection and co-localizes with host cell chromosomes, and plays an important role in episomal maintenance by tethering viral genomes to host cell chromosomes [9]. It can be found in nearly all infected cells in KS, as well as in PEL and MCD [11,12]. And the antibodies to the latent nuclear antigen-1 are highly specific for persistent KSHV infection, for it has no homology to other known human herpesviruses [15,17]. It was also reported that antibodies to the latent nuclear antigen were detected in all 12 sera obtained from patients with classical KS by the immunofluorescence assay, and nine of them were positive in ELISA [10].
HTML
-
TA cloning vector pGM-T, prokaryotic expression vector pQE-80L, and Ni-NTA resin were purchased from QIAGEN Co., the E.coli DH5a and BL21 (DE3) strains were kept in our lab. DNA restriction enzyme and supporting buffer, Taq DNA polymerase, dNTP, and T4 DNA ligase were purchased from TaKaRa Co.
-
Based on the gene sequence of KSHV orf73 (GenBank accession No: NC003409), primers were designed and named P1 and P2 (P1: 5'-GTGGGATCCGATTACCCTGTTGTTAGCACA-3', P2: 5'-AGCGTCGACTTATGTCATTTCCTGTGGAGA-3'), for amplifying 651 bp orf73 fragment encoding the c-terminal of KSHV ORF73.
-
BCBL-1 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100U/mL penicillin and 100 mg/mL streptomycin and cultured at 37℃ in a 5% CO2 incubator. KSHV genomic DNA was extracted from BCBL-1 cells in logarithmic growth phase using the Hirt method and used as the PCR template. PCR was performed over 30 cycles of 94℃ 30 s, 60℃ 30 s and 72℃ 30 s, followed by an extension at 72℃ for 5 min. Then, five microliters of the PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
-
The PCR product was ligated into pGM-T vector for constructing recombinant plasmid pGMT-orf73 and transformed into DH5α competent cells for amplification. Then, the recombinant plasmid was extracted from DH5α cells, and the truncated orf73 gene was removed by the digestion of Sal Ⅰ and BamH Ⅰ from pGMT-orf73 and confirmed by sequencing and subcloned into pQE-80L plasmid. The latter recombinant plasmid was named as pQE80L-orf73, and used for expressing the recombinant ORF73 in BL21 (DE3) cells.
-
The pQE80L-orf73 was transformed into BL21 (DE3) competent cells and incubated in 5 mL LB medium with 50 mg/mL ampicillin at 37℃. When the culture's OD600 value reached approximate 0.6, IPTG was added to the final concentration of 1mmol/L. The culture was further incubated at 37℃ for another 6 h. Then, the cells were collected by centrifugation at 5 000 r/min for 10min.
-
The cells were resuspended by 10 × volume PBS, disrupted by ultrasound (250w, 3×5 second burst) for 5 min, added with 20% Triton X-100 to a final concentration of 1%, stirred for 30 min at room temperature, and then centrifuged at 10 000 r/min for 10 min. Then, the supernatant and the precipitate was collected separately, mixed with SDS-PAGE Gel loading buffer, boiled for 3 min, then loaded onto 10% SDS-PAGE Gel and electrophoresed at 100 V. Using Ni-NTA Agarose according to the manufacturer's instructions, the recombinant ORF73 was purified.
-
Proteins of the whole cell lysate including both before and after IPTG induction, and the purified recombinant ORF73 were loaded on a SDS-PAGE gel for electrophoresing as described elsewhere [5]. Proteins were visualized by Coomassie brilliant blue R250 staining.
-
The ORF73 proteins separated by SDS-PAGE were transferred by electroblotting onto a polyvinyllidene difluoride (PVDF) membrane. The membrane was washed and blocked by the blocking solution (PBS-T) containing 0.2% Tween-20, then incubated with KSHV positive serum called S558 or KSHV negative serum called H14 as the primary antibody [6]. The membrane was washed again in PBS-T and incubated at room temperature for 1 hour with anti-human IgG-HRP antibody (1:10 000 dilution). The bands were detected by an Alpha Innotech MultiImageTM system after incubation in Chemiluminescent Substrate (Pierce).
-
ELISA plates were coated with 50 mL purified ORF73 with different concentrations (1 mg/mL, 2 mg/mL, 5 mg/mL and 10 mg/mL) in 0.1 mol/L NaHCO3 at pH 9.6 overnight at 4℃. A conventional ELISA protocol was used with phosphate-buffered saline containing 0.05% Tween 20 (PBS-T, pH 7.4) for washes, using 5% dried skimmed milk in PBS-T (blocking buffer) to saturate plates and to dilute S558 or H14 (from 1:100 to 1:1 000), which was characterized as KSHV positive and negative serum respectively [1], and an alkaline phosphatase conjugated affinity purified goat anti-human IgG, diluted 1 in 1 000 in blocking buffer, followed by 1 mg/mL of nitrophenyl phosphate in glycine buffer (0.1 mol/L glycine, 1 mol/L MgCl2, 1 mmol/L ZnCl2, pH 10.4) as substrate. The colorimetric reaction was stopped after 25 min at 37℃ with 100 µL 3 mol/L NaOH and read spectrophotometrically at 405nm.
-
To check the sensitivity and specificity of ORF73, sera from 20 KS patients and 50 blood donors that were determined KSHV negative in a previous study were used in ELISA[4,5]. Cutpoint was set to be the mean OD of negative controls plus five standard deviations of the negative controls[17].
-
430 sera from general Han population were collected from June to July of 2007 by the Laboratory of Xinjiang Endemic and Ethnic Disease (Shihezi University). 560 sera from general Hubei Han people were collected from April of 2004 to April of 2005. ORF73 ELISA was used to detect KSHV infection ratio.
-
The seroprevalence of KSHV and corresponding 95% confidence intervals (CI) were calculated using standard epidemiologic methods. Odds ratios (OR) and the 95% confidence intervals were used to quantify the relationships in estimates while P-values were calculated to indicate the statistical significance. CI was calculated based on coefficients and standard errors from the logistic model. A P-value less than 0.05 were considered to be significant.
Main reagents and materials
Primer designing
Amplification of ORF73 gene by PCR
Construction of prokaryotic expression plasmid pQE80L-orf73
Expression of orf73
Purification of ORF73
SDS-PAGE analysis
Western blot analysis
Optimization of the condition of ELISA
Evaluation of the recombinant ORF73 in ELISA
Comparison of KSHV infection ratio between Hubei Han people and Xinjiang Han people
Statistical analysis
-
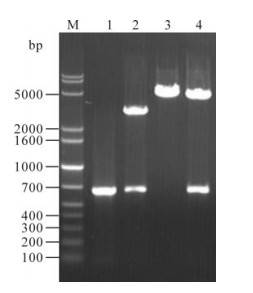
A fragment of 651 bp was obtained by PCR with specific primers and cloned into pGMT-easy vector. The 651 bp fragment was identified as c-terminal orf73 gene by restriction endonuclease cleavage and sequence analysis. Then the orf73 gene fragment was subcloned into the pQE-80L vector to obtain the prokaryotic expression plasmid pQE80L-orf73. Plasmid pQE80L-orf73 was then transformed into BL21 (DE3) and also identified by digestion of Sal Ⅰ and BamH Ⅰ.(Fig. 1).
-
Total cell proteins were attained from BL21 (DE3) transformed by pQE80L-orf73 both before and after IPTG induction. Proteins in the supernatant and the precipitate were obtained by centrifugation. Recombinant ORF73 protein was purified by Ni2+-NTA acid resin affinity chromatography. All proteins mentioned above were separated by SDS-PAGE (Fig. 2). SDS-PAGE showed that recombinant ORF73 protein was approximately 27 kDa as expected and was highly expressed in BL21 (DE3) cells after the induction of IPTG. The separation of supernatant and precipitate indicated that ORF73 had a higher concentration in the precipitate of the lysed BL21 (DE3) transformed by pQE80L-orf73 than that in the supernatant. By using bandscan software, the purity of recombinant ORF73 protein was analyzed and found to be as high as 99%.
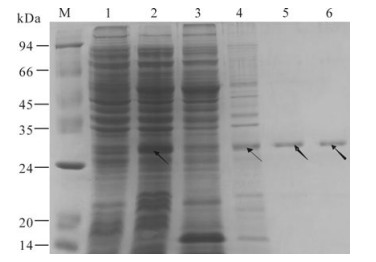
Figure 2. Analysis of the expression of recombinant ORF73 protein. Lane 1, The lysate of BL21 (DE3) transformed with pQE80L-orf73 before IPTG induction; 2, The lysate of BL21 (DE3) transformed with pQE-80L-orf73 after IPTG induction; 3, The supernatant of the lysed BL21 (DE3) transformed with pQE-80L-orf73 induced with IPTG; 4, The precipitate of the lysed BL21 (DE3) transformed with pQE-80L-orf73 induced with IPTG; 5 and 6, Purified ORF73 protein; M, protein marker. Arrow indicates the expressed ORF73.
-
Proteins of the whole BL21 (DE3) cells transformed with pQE80L-orf73 both before and after IPTG induction and the purified recombinant ORF73 were electrophoresed by SDS-PAGE and transferred to a PVDF membrane. Then, the PVDF membrane was coated with the KSHV positive serum or negative serum (Fig. 3). The Western blot results showed that a specific 27 kDa band occurred in the lanes of the total protein of lysed BL21 (DE3) transformed with pQE80L-orf73 after the induction of ITPG, and the purified recombinant ORF73, when they were incubated with KSHV positive serum; no band occurred when incubated with serum from healthy blood donors. The results above revealed that the antigenicity of recombinant ORF73 was quite strong.
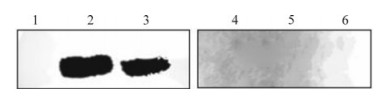
Figure 3. Western blot of recombinant ORF73. Lane 1 and 4, The lysate of BL21 (DE3) transformed with pQE-80L-orf73 before IPTG induction; 2 and 5, The lysate of BL21 (DE3) transformed with pQE-80L-orf73 after IPTG induction; 3 and 6, Highly purified recombinant ORF73; 1, 2 and 3, Reacted with KSHV positive serum; 4, 5 and 6, Reacted with negative serum.
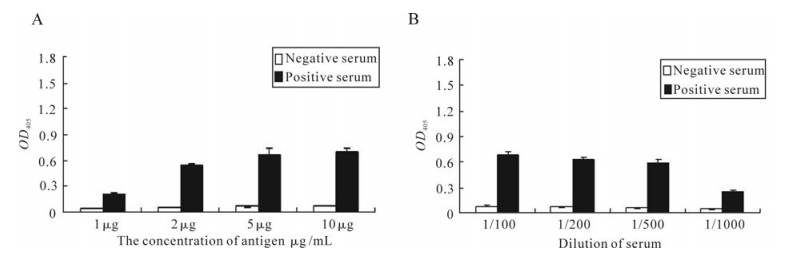
ELISA was also used to check the antigenicity of the recombinant ORF73 protein. The recombinant ORF73 was used as antigen to coat the ELISA plates; KSHV positive and negative serum were used as the primary antibody separately (Fig. 4). The results not only showed that the antigenicity of the recombinant ORF73 recombinant protein was strong, but also revealed that ELISA using the recombinant ORF73 was a good choice for screening a large amount of sera for epidemiological research.
-
By using different concentrations of recombinant ORF73 protein and different dilutions of serum, the optimal condition of ELISA was investigated (Fig. 4). As the ELISA results showed, all the chosen concentration of recombinant ORF73 could show the difference between negative and positive serum; at concentrations of 10 µg/mL and 5 µg/mL, the values of OD405 were higher than 0.6; when the concentration declined to 2µg/mL, the value of OD405 was about 0.6; while at the concentration of 1 µg/mL, the OD405 value was lower than 0.3 (Fig. 4A). As to the optimal dilution of the serum in ELISA, it showed that all four different dilutions could distinguish the difference between negative and positive serum. The OD405 values of the dilution of 1:100 and 1:200 were both higher than 0.6; when the dilution became 1:500, the OD405 value declined to almost 0.6; while at the 1:1 000 dilution, the OD405 value became lower than 0.3 (Fig. 4B). Thus, 2 mg/mL recombinant ORF73 and 1:100 dilution of the serum will be used in ELISA in the future KSHV epidemiological research.
-
To determine the specificity and sensitivity of the recombinant ORF73 in detecting KSHV in sera, 20 sera from KS patients collected by the Laboratory of Xinjiang Endemic and Ethnic Diseases and 50 healthy subjects which were characterized as negative for KSHV [5,6]were detected for KSHV infection. Overall, the recombinant ORF73 could detect 20 KS samples as KSHV positive, and 50 healthy subjects as negative. Thus, the recombinant ORF73 had a combined specificity of 100% and sensitivity of 100% (Table 1).

Table 1. Specificity and sensitivity of the recombinant ORF73
-
Of 560 subjects from the general Han population in Hubei, 16 (2.9%) were KSHV-positive. KSHV seroprevalence in this population was compared with the general Han population in Xinjiang, a region known to have high KSHV seroprevalence. Of 430 subjects from the general Han population in Xinjiang, 29 (6.7%) were KSHV-seropositive (Table 2). Logistic regression analysis showed that the Han people in Xinjiang had 146% increase in their risk for KSHV infection compared to their counterparts in Hubei (6.7% vs 2.9%, OR: 2.46, 95%CI: 1.32-4.59, P = 0.005) (Table 2).

Table 2. Logistic regression analysis of KSHV seroprevalence in different Han population from Hubei and Xinjiang
Construction of prokaryotic expression plasmid
Expression and purification of recombinant ORF73 protein
Antigenicity identification of the recombinant ORF73 protein
Optimization of the condition of ELISA
Specificity and sensitivity of the recombinant ORF73 in ELISA
KSHV seroprevalence in Hubei and Xinjiang Han people
-
KSHV is an ancient virus and now thought to be essential for the development of all forms of four clinical epidemiological variants of KS, including AIDS KS, classic KS, endemic KS and iatrogenic KS. The prevalence of KSHV has been extensively investigated in the last decade. The seroprevalence of KSHV is relatively low in the general population in North America and Europe, ranging from 0-15% [8,16]. In Mediterranean and East European regions, KSHV prevalence is between 4-24% [8,14]. In sub-Saharan Africa, KSHV seroprevalence is high, ranging from 30-70% [16]. In Asia, KSHV seroprevalence is usually low. In Japan, it ranges from 0.14% to 0.20% [7]. Recently, limited studies have been done to investigate the seroprevalence of KSHV in China. A generally low KSHV seroprevalence in the range of 0.5%-7.3% in most parts of China has been reported. However, in Xinjiang where there is high incidence of KS, it is 19.2% [6]. KS is rare in the Han Chinese, but in Xinjiang Uygur Autonomous Region, there is a high incidence of classic KS, especially in the Uygur ethnic group [21]. Recently, as the number of HIV-1 infection in China is rapidly increasing, the number of AIDS KS cases also increases. So it is important to study the epidemiology of KSHV infection in China.
The development of serological assays capable of detecting antibodies against KSHV has proved effective for large-scale epidemiological studies, which have provided important information on KSHV infections [3]. Four different serologic assays including enzyme-linked immunosorbent assays (ELISA), immunofluorescent assays (IFA), Western blot and immunohistochemistry (IHC) have been used to detect the KSHV antibodies. Among them, ELISA is the optimal choice for screening a large amount of sera for epidemiological research [5].
In the present study, a 651 bp c-terminal DNA fragment of KSHV orf73 gene was cloned and inserted into the prokaryotic expression vector pQE-80L to obtain the expression plasmid named pQE-80L-orf73. After the recombinant plasmid was transformed into BL21 (DE3) strain, recombinant ORF73 protein was induced to express by IPTG, and purified by Ni2+-nitrolotriacetic acid resin affinity chromatography from the whole cell lysate. The purity of recombinant ORF73 could reach 99% and the SDS-PAGE results showed ORF73 was approximately 27 kDa as expected. Western blot was used to check the antigenicity of the expressed ORF73. As shown in Fig. 3, the specific ORF73 bands appeared when reacted with KSHV positive serum, while treated with negative serum, the bands did not appear. The results indicated that the expressed ORF73 proteins could be used to detect antibodies to LANA-1 in sera.
The sensitivity and specificity of the expressed recombinant ORF73 was determined by using ORF73 as antigen to react with 50 healthy sera and 20 KS patient sera. The data showed that the recombinant ORF73 had a combined specificity of 100% and sensitivity of 100% (Table 1). The data suggest that the expressed recombinant ORF73 is highly antigenic and specific in immunoreaction.
We use the ORF73 ELISA to compare the KSHV seroprevalence between Hubei Han people and Xinjiang Han people. Interestingly, the Han people in Xinjiang (6.7%) have significantly higher KSHV seroprevalence than their counterparts (2.9%) in Hubei (Table 2) (P = 0.005). In Hubei, where KSHV seroprevalence is low, Han people have relatively low KSHV infection ratio. In Xinjiang, the minority ethnic groups such as Uygur and Hazakh people have a relatively high KSHV infection ratio. Most Han people in Xinjiang have only immigrated to this region in the last 50 years; they might attract new infection after migrating to the region and exposing to the endemic population.
The successful expression of KSHV ORF73 with strong antigenicity and the determination of optimal ORF73 ELISA condition will facilitate the diagnoses of KSHV infection. Combined with our antecedent work, we have established a KSHV diagnostic method by detecting antibodies of ORF73, ORF65, and K8.1 using ELISA. This method can be used in KSHV epidemiological research and be helpful for prevention and control of KSHV infection in China.











 DownLoad:
DownLoad: