-
Herpes simplex virus type 1 (HSV-1), a typical alphaherpesvirus, is a common human pathogen that is associated with infections of the mucocutaneous membranes, brain, and internal organs of infected neonates[8]. The HSV-1 genome is a 152-kilobase (kb) linear duplex DNA molecule, and 84 open reading frames were expressed in the infected cells. Expression of the viral genes occurs in a coordinately activated cascade that consists of the sequential expression of immediate-early (IE), early (E), and late (L) genes[10]. Infected-cell protein 27 (ICP27), a 63 kDa immediate-early regulatory phosphoprotein, is an essential, highly conserved protein and has been extensively studied[16]. ICP27 is involved in various steps of HSV-1 gene regulation as well as in the shut-off of host gene expression during infection. Recently, many novel functions have been reported for the ICP27 protein, including inhibition of the IFN-1 signaling[6], regulation of the viral mRNA translation[3] and determining the composition of HSV-1 virions[17].
In this study, we constructed a recombinant expression plasmid that drives expression of the HSV-1 ICP27 fused with His6. The recombinant protein His6-tagged ICP27 was expressed in E. coli BL21 (DE3) cells. His6-tagged ICP27 was purified by single-step immobilized metal affinity chromatography (IMAC) on a nickel-nitrilotriacetic acid (Ni2+-NTA) affinity resin and applied to raise the antiserum in rabbit. The antiserum, shown to be sensitive and specific by Western blot and immunofluorescence analysis, can be applied to further characterize the biological function of ICP27 during HSV-1 infection.
HTML
-
African green monkey kidney (Vero) cells were cultured in minimal essential medium (MEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL). For virus infection, MEM supplemented with 2% FBS was applied and HSV-1 strain F was used for the infection of Vero cells.
-
pET-28a (+) and E. coli BL21 (DE3) were purchased from Novagen (Madison, WI). All restriction endonucleases and DNA polymerase were from New England Biolabs (Ipswich, MA). The nickel-nitrilotriacetic acid (Ni2+-NTA) affinity resin column was supplied by Roche Diagnostics (Mannheim, Germany). Complete Protease Inhibitor Cocktail was purchased from Roche Diagnostics (Indianapolis, IN). Three month-old New Zealand white rabbits for antiserum production were purchased from the Laboratory Animal Centre, Wuhan Institute of Virology, Chinese Academy of Sciences.
-
pYEbac102[18] was used as a template to amplify the ICP27 open reading frame from full-length HSV-1 (F) genome with the forward primer (5'-TTGAATTCATGGCGACTGACATTGATA TGC-3') including an EcoR Ⅰ restriction site (underlined), and the reverse primer (5'-CCG CTCGAGAAACAGGGAGTTGCAATAAA-3') including an Xho Ⅰ restriction site (underlined). The PCR fragment was digested with EcoR Ⅰ and Xho Ⅰ and inserted into the prokaryotic expression vector pET-28a (+), digested with the same enzymes to generate the plasmid pET28a-ICP27.
-
E.coli BL21 (DE3) cells were transformed with the plasmid and the expression of the recombinant protein was induced by isopropyl-β-D-thiogalactopyranoside (IPTG) at 1.0 mmol/L when the culture reached an A600 of 0.6. To determine the solubility of the recombination protein, the induced cells were suspended in PBS and sonicated. The soluble and insoluble fractions were then analyzed in parallel by 10% SDS-PAGE.
-
The cells from 100 mL of culture medium were harvested and resuspended in 30 mL binding buffer (50 mmol/L Na3PO4, 300 mmol/L NaCl, 10 mmol/L imidazole, pH 8.0) containing complete protease inhibitor cocktail and subjected to sonication. The recombinant protein was purified by immobilized metal-ion affinity chromatography (IMAC) on a nickel-nitrilotriacetic acid (Ni2+-NTA) affinity resin according to the manufacturer's instructions. Then the purity of the protein was analyzed by 10% SDS-PAGE, and the concentration was estimated with an ultraviolet spectrophotometer (Bio-Rad).
-
The antiserum against the recombinant protein was raised in New Zealand white rabbits. Briefly, preimmune sera were collected before immunization. The recombinant protein (800 μL, 0.5mg/mL) was mixed with an equal volume of Freund's complete adjuvant (Sigma) and subcutaneously injected into the footpad and hindquarter of the rabbit. The rabbits were immunized three times with an interval of 14 days, and the antiserum was harvested from the arteriae carotis.
-
To evaluate antibody titers, three independent enzyme linked immunoabsorbent assays (ELISA) were performed as described elsewhere[14]. Briefly, the purified protein with 0.5 mg/mL concentration was coated with coating buffer in a 96-well plate overnight. The coated plate was washed 3 times with washing buffer, and then the plate was incubated with a series of dilutions of the anti-ICP27 serum. After another triple wash, a 1:2 000 dilution of goat anti-rabbit alkaline phosphatase labeled secondary antibody (Sigma) was added. Finally, the color was developed with NBT/BCIPand the OD value was read at 450 nm. The 1×PBS and normal rabbit serum were used as negative controls.
-
The purified recombinant protein was subjected to 10% SDS-PAGE, and the gel was electrophoretically transferred to a nitrocellulose membrane. The rabbit antiserum against the recombinant protein was used as the primary antibody and alkaline phosphatase (AP)-labeled goat anti-rabbit IgG (Sigma) was used as the secondary antibody.
-
Vero cells were cultured to 90% confluence on glass coverslips followed by infection at a MOI of 10 with HSV-1 strain F. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and incubated with the antiserum and FITC-conjugated goat anti-rabbit IgG (Zymed Laboratories). The cells were visualized with a Zeiss Axio Observer A1 microscope (Carl Zeiss, Germany). Images were processed with Adobe Photoshop.
Cells and virus
Animals and reagents
Recombinant plasmid construct
Expression of recombinant protein
Purification of recombinant protein
Antiserum production for the recombinant protein
ELISA assay
Western blot analysis
Immunofluorescence assay
-
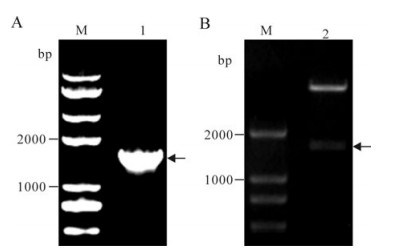
The ICP27 gene was successfully amplified, and EcoRⅠand XhoⅠ digested PCR product was inserted into the vector pET-28a (+) digested with the same restricted enzymes to construct the recombinant plasmid pET28a-ICP27. Then the pET28a-ICP27 was verified by colony PCR (Fig. 1A) and restriction digestion (Fig. 1B). The nucleotide sequence reported in this paper has been submitted to the GenBank nucleotide database under accession number GQ386863.
-
SDS-PAGE analysis showed that the expressed recombinant protein could be detected in soluble fraction of the induced cells but not in cells without induction (Fig. 2A). A distinct band of approximately 70 kDa corresponds to the expected His6-tagged ICP27 fusion protein.
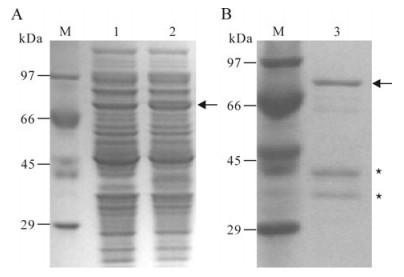
Figure 2. Expression (A) and purification (B) of the recombinant protein. A: M, Standard protein marker; 1, Extracts of E. coli transformed with the recombinant plasmid and non-induced; 2, Extracts of E. coli transformed with the recombinant plasmid and induced with IPTG. B: M, Standard protein marker; 3, the purified recombinant protein. Arrows indicate the induced recombinant protein ICP27. Asterisks indicate the degraded ICP27.
-
Purification of His6-tagged ICP27 was performed in a Ni2+-NTA resin column, with the SDS-PAGE analysis showing that the target protein could be conjugated to the resin and the elute contained a band of target protein with a molecular weight of 70 kDa as well as two other bands corresponding to the degraded protein (Fig. 2B).
-
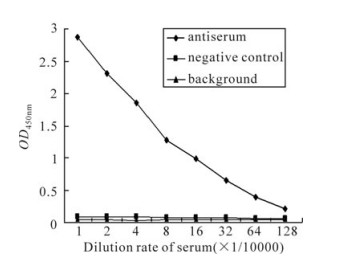
The recombinant protein was applied to raise antiserum in New Zealand white rabbits. A high titer of antiserum was detected after the third injection by the ELISA assay (Fig. 3), which indicated that the recombinant protein ICP27 has a good immunogenicity and the antiserum is specific to the recombinant protein. Western blot analysis showed that the antiserum could react with the purified protein ICP27 (Fig. 4). Moreover, as shown in immunofluorescence assay, the antiserum could identify the ICP27 protein expressed in HSV-1 infected cells (Fig. 5A) and the ICP27 was distributed within the nucleus consistent with a previous report[12], whereas no immunofluorescence was observed from mock infected cells (Fig. 5B), and no immuno-fluorescence was observed with the pre-immune serum (data not shown).
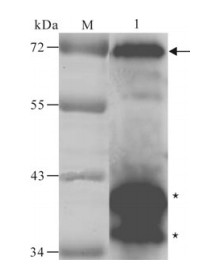
Figure 4. Western blot analysis of the purified recombinant protein with the specific antiserum.M, low range pre-stained protein marker; 1, the purified recombinant protein. The arrow indicates the purified ICP27 fusion protein; Asterisks indicate the degraded ICP27.
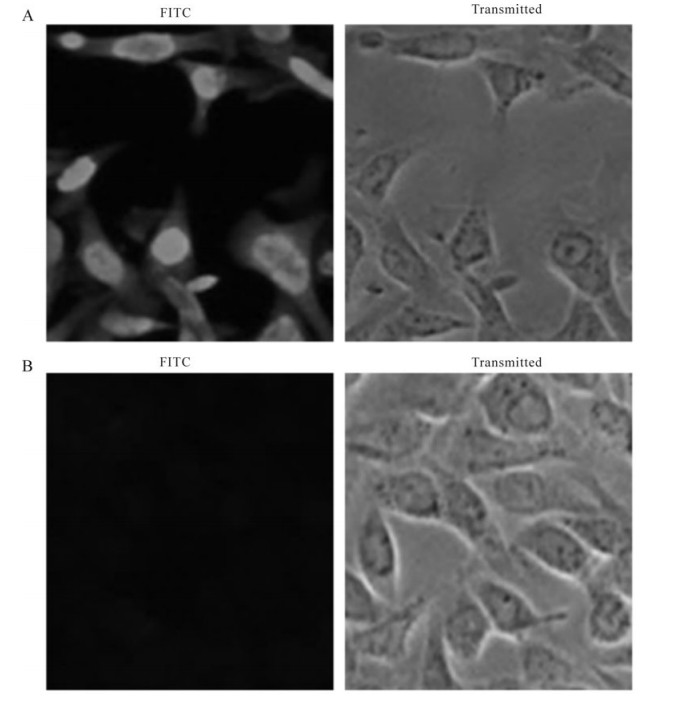
Figure 5. Immunofluorescence assay of the recombinant protein in HSV-1-infected Vero cells. HSV-1 strain F and mock infected cells were fixed with 4% paraformaldehyde at 12 h post-infection and subjected to immunofluorescence assay with the specific antiserum. A: HSV-1 strain F infected Vero cells with the antiserum. B: Mock infected Vero cells with the antiserum.
Gene amplification, cloning, and sequencing
Expression of His6-tagged ICP27
IMAC purification of the His6-tagged ICP27 recombinant protein
Production of antiserum specific to the His6-tagged ICP27 recombinant protein
-
HSV-1 can be used as a model system and to develop tools for the study of the translocation of proteins, membrane structure, gene regulation, gene therapy & cancer therapy. HSV-1 ICP27, a multifunctional regulatory protein, is conserved in all mammalian and avian herpesviruses, and is required for HSV-1 productive infection. ICP27 has been implicated in all aspects of gene expression from transcription through RNA processing and export to translation. Depending on the target gene, ICP27 is responsible for both repression and transactivation of viral gene expression at a post-transcriptional level. It is reported that ICP27 inhibits pre-mRNA splicing[4], stimulates pre-mRNA 3′ processing[11], affects mRNA stability[2], and shuttles between the nucleus and the cytoplasm[13, 15], promoting viral RNA nuclear export[7, 15]. The function of ICP27 to promote translation of viral mRNA has been identified recently[3]. ICP27 is also involved in the inhibition of type 1 interferon signaling[6] and determining the composition of HSV-1 virions[17]. Although much has been learned about the mechanisms of the roles of ICP27, relatively little is known about how its activities are regulated. Here, the specific antiserum, produced by our lab, against His6-tagged ICP27 can serve as a valuable tool for further studies investigating ICP27's biological function during HSV-1 infection.
There are a variety of reliable expression systems for large-scale recombinant protein production, the bacterial expression system recognized for its ability to rapidly and effectively produce recombinant protein, is the most universal protein expression systems[9]. E. coli BL21 (DE3) is the preferred host, in which protein production offers several advantages over other mostly eukaryotic hosts such as short generation time, well-established methods for genetic manipulation, and simple, inexpensive cultivation[5]. pET-28a (+) containing a polyhistidine (His6) tag was selected as expression vector, because this His-tag, as the most extensively used affinity tag, exhibits a high binding capacity allowing for a very rapid and single-step purification[1]. Consequently, in this study, ICP27 was constructed as a fusion protein with a His6-tag to purify the recombinant protein.
Some proteins are easy degraded, and this was observed for ICP27 in the purification process. Many measures were taken to block the degradation of ICP27; for example, the inhibitor cocktail was added two times at the recommended concentration, the whole purification process was under 4℃ and the pH value of the purification buffer was adjusted. Following these modifications, high quality antiserum was obtained using the purified recombinant ICP27 protein. Western blot analyses demonstrated that the antiserum reacted with the purified ICP27 protein indicating the antibody shows high reactivity and specificity. Immunofluorescence assay showed that the antiserum could recognize the ICP27 in the HSV-1 infected cells. Taken together, the antiserum will be valuable tool for studying the function of ICP27 during HSV-1 infection.













 DownLoad:
DownLoad: