-
Porcine reproductive and respiratory syndrome (PRRS) was first observed in America in 1987, and subsequently spread worldwide, becoming one of the main infectious diseases in the swine industry and the cause of substantial economical losses. PPRS is characterized by pregnant sow reproductive failure, highly mortality in piglets and respiratory symptoms in growing pigs [1]. The PRRS virus (PRRSV), is a small enveloped virus with a single-stranded RNA genome containing eight ORFs. These are the viral replicase encoded by ORF1, which contains ORF1a and ORF1b; the virus envelope glycoproteins GP2, GP3, GP4 and GP5 encoded by ORFs 2-5 respectively [2]; the viral unglycosylated membrane protein M encoded by ORF6; and the highly conserved nucleocapsid protein N encoded by ORF7 [4].
GP5 protein is a major multifunctional glycoprotein of 25 kDa. It has six antigenic determinants that can induce the production of antibodies that neutralize viral infection in vitro [6]. GP5 shows the greatest variability of the PRRSV proteins, and stimulates cellular and humoral immunity. Therefore, establishing methods for detection of mAbs using GP5 protein as the antigen is feasible.
Monoclonal antibodies (mAbs) play an important role in infectious disease research, diagnosis and therapy. The largest area of mAb application in virology has been in diagnosis. The use of mAbs increases the specificity, accuracy and efficiency of diagnostic tests compared to polyclonal anti-sera and provides unlimited quantity and consistent quality of reagent supplies. In this study, we obtained a specific mAbs against PRRSV, which may serve as a useful tools in future studies of PRRSV and development of diagnostic kits for the detection of PRRSV.
HTML
-
PRRSV LZh/07 strain, Marc-145 cells and recombinant E. coli BL21 [11] used in this study were preserved at the Virology Department of Lanzhou Veterinary Research Institute. Complete Freund's adjuvant, incomplete Freund's adjuvant, Horseradish peroxidase, 3-(4, 5-Dimethylthiazoyl2-yl)-2, 8-azaguanine, Tetramethylbenzidine and Dimathyl sulfoxide (DMSO) were purchased from Sigma Chemical Co., USA. SP2/0 cells were purchased from ATCC (Manasa, VA).
-
The GP5 fusion proteins [11] were purified by resuspending E. coli cell pellets in a 0.1×culture volume of inclusion body (IB) wash buffer (20 mmol/L Tris-HCl, pH 7.5; 10 mmol/L EDTA; 1% Triton X-100) supplemented with lysozyme to a final concentration of 100 mg/mL. The mixture was incubated at room temperature for 15 min and sonicated on ice until no longer viscous. Inclusion bodies were collected by centrifugation at 10 000 × g for 10 min, washed twice with IB washing buffer, and once with a second wash buffer (20 mmol/L Tris-HCl, pH 7.5; 2 mol/L urea; 1% Triton X-100). Inclusion bodies were solubilized by the addition of IB solubilization buffer (50 mmol/L CAPS, pH 11; 0.3% N-lauroylsarcosine; 1 mmol/L DTT). Fusion protein was purified with a GST•Bind™ Purification Kit (Novagen), according to the manufacturer's instructions. The purified fusion proteins were used as antigens for Western blotting and ELISA for detecting PRRSV antibodies.
-
Female BALB/C mice of 5-6 weeks old were immunized with of inactivated PRRSV LZh/07 in an equal volume of complete Freund's adjuvant subcutaneously. Three identical boosters emulsified in incomplete Freund's adjuvant were given at 4 weeks interval. Mice were boosted with the same antigen in PBS by intraperitoneal injection 3-4 days before fusion. The two immunized mice used for each fusion were sacrificed by overdose anaesthesia. A single-cell splenocyte suspension was obtained for fusion. DMEM with 10% fetal bovine serum was used for fusion and subcloning. Immunized spleen cells were fused with myeloma cells at 5-10:1 ratio in the presence of 50% polyethylene glycol (Merck). The cells were plated out in semisolid medium (Stem Cell) and incubated at 37℃ in a humidified 5% CO2 atmosphere [3]. After two weeks, single colonies were transferred to 96-well culture plates. Hybridoma supernatants were screened using either an indirect ELISA or a double antibody sandwich ELISA. The positive hybridomas were subcloned using the limiting dilution technique [5]. The mAb isotyping was performed using a mouse monoclonal antibody isotyping kit (Isostrip, Roche) according to manufacturer's instructions.
-
The isotype of two mAbs was determined by adding 25 μL of the cell culture supernatant containing 8C9 and 4B4 respectively with 200 μL assay buffer to wells coated with each of the rabbit anti-mouse antibodies from a mouse MonoAb ID kit (Zymed, San Francisco, CA) against IgG1, IgG2a, IgG2b, IgG3, IgA, IgM. Detection of bound mAb was by goat anti-mouse IgG-HRP conjugated antibody (Zymed, San Francisco, CA). IEF was performed in IEF gel pH 3-7 (Invitrogen). Electrophoresis was run following the manufacturer's protocol.
-
A ninety-six-well flat-bottom plate was coated with serially diluted PRRSV GP5 recombinant protein. Plates were then washed three times with PBS containing 0.05% Tween 20. Non-specific sites were blocked with 1% BSA at 37℃ for 2 h. Three mAbs were added (100 ng/well) and incubated at 37℃ for 2 h. After washing with Tween/PBS, an HRP-conjugated goat anti-mouse IgG (Sigma) was used for detection.
-
The purified GP5 fusion proteins were used as antigens for Western blotting and ELISA for detecting PRRSV 8C9 and 4B4 mAbs. Purified GP5 was separated on 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad), incubated for 1 h in blocking solution (5% non-fat dry milk, 0.1% Tween-20 in phosphate buffered saline, PBS) and probed with 8C9 and 4B4 mAbs diluted 1:10 000 in blocking solution. Specificity was detected with protein goat anti-mouse with horseradish peroxidase conjugate (Sigma) and the reaction developed with luminol.
-
The microneutralization (MN) assay procedure for the detection of mAb neutralization activity were followed with modification. Both serial diluted mouse ascitic fluid and equal volume of 100 TCID50 of the LZh/07 virus prepared with 2% FCS in MEM were mixed and incubated at 37℃ for 1 hr. Each virus/ diluted mAb mixture was then applied to eleven wells of a 96-well microtiter plate. Marc-145 cells 5×104 0.1 mL/well were also added. After incubation for 7 days at 37℃, the fifty percent end point of neutralization titers for LZh/07 were calculated. The amount of virus actually used per well should contain 100 TCID50 within an acceptable range (e.g.35-350 TCID50) from back titration control. Neutralization assays were repeated at least two times.
-
In this section, the specificity, stability and titers of mAbs were examined by ELISA (using the procedure as described previously [11]). For the specificity, two mAbs were tested for reactivation with CSFV and SVDV. The stability and titers of prepared mAb were be estimated by ELISA in different passaged generations.
Materials
Preparation of PRRSV recombinant GP5
Mice immunization and generation of hybridoma cells
Isotying and isoelectric focusing
ELISA
mAbs SDS-PAGE analysis
Neutralization assay
mAbs characteristic analysis
-
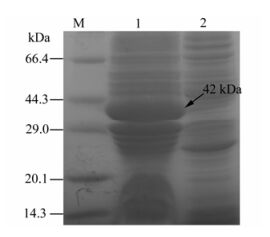
The results showed a specific protein band with molecular weight of 42 kDa appeared after induction at 28 ℃ and 12h, which was consistent with the expected results. The analysis results suggested that the recombinant GP5 protein content accounted for 30% of the total thallus protein content. (Fig. 1).
-
mAbs were produced from mice immunized with PRRSV lzh/07, and the two specific clones described here were named 8C9 and 4B4. The purified monoclonal antibody by SDS-PAGE showed the molecular weights of the heavy chain and light chain were about 45.0 kDa and 25.0 kDa respectively (Fig. 2A), which was consistent with the predicted molecular weight.

Figure 2. SDS–PAGE and Western blot analysis of the purified mAbs 8C9 and 4B4. A: SDS–PAGE analysis. Lane 1 and 2, Purified mAb 8C9 and 4B4 respectively. Lane M is the protein marker. B: Western blot analysis of the purified mAb 8C9 and 4B4. Lane 1and 2, Purified mAb 8C9 and 4B4 respectively. Lane M is the protein marker.
Purified recombinant GP5 (0.5 μg/well) were separated on 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad), incubated for 1 h in blocking solution (5% non-fat dry milk, 0.1% Tween-20 in phosphate buffered saline, PBS) and probed with a mAbs 8C9 and 4B4 diluted 1:5 000 in blocking solution. Specific anti-PRRSV antibodies were detected with protein A-horseradish peroxidase conjugate (Sigma) and the reaction developed with luminol. Affinity chromatography-purified GP5 proteins were stored at -70℃ for use as ELISA antigens (Fig. 2B).
-
The neutralization activities of prepared mAbs were shown in Table 1. In this test, the setting virus control, mAb control and cell control were normal until the time end, the result showed that the prepared 4B4 mAbs can protected 50% cell no CPE in the dilution reach to 1:512. But 8C9 can't neutralized ability to PRRSV. This indicated that 4B4 have neutralization activity, 8C9 have no this ability.

Table 1. Detection of mAbs neutralization titers by virus neutralization test
-
The two mAbs, 4B4 and 8C9, which showed the highest binding activity for PRRSV were selected and characterized, and found to specifically bind to PRRSV without cross-reactivity with SVDV and CSFV (Table 2). In an isotype test, 8C9 was found to be of type IgG1, whereas 4B4 belonged to type IgG2a. Their titers in cell culture supernatant were 1:104, and the titers of abdomen liquor were 1:4×105. In stability tests, the titers of prepared mAbs were maintained invariably when passaged to thirty generations (data not shown). All of these results showed that the selected mAbs possessed good specificity and high titers.

Table 2. The result of the cross-reaction test
Purification of PRRSV GP5 protein
Purification of mAb 8C9 and 4B4 and western-blotting analysis
Neutralilzation assay
Characterasty of the mAbs
-
Porcine reproductive and respiratory syndrome (PRRSV) encodes seven kinds of structure proteins, of which the ORF5 gene has the highest variability [2, 11]; the ORF5 gene in strains isolated from different countries shows significant genetic diversity, for example, the nucleotide homology between the ORF5 gene from Europe and America is only 54%. Furthermore, PRRSV can mutate in multiple loci of GP5 during proliferation in pigs [4, 7]. Thus, studying the PRRSV ORF5 gene has become the key method for deriving an effective PRRS vaccine.
GP5 is a glycosylated envelope protein, which is one of three major structure proteins for the virus and has good immunogenicity, so it can induce the secretion of neutralizing or non-neutralizing antibodies [6]. Additionally, the antiserum mainly identifies GP5 protein after appearance of non-neutralizing antibodies and other studies suggest that GP5 probably takes part in the process by which the virus receptor is coupled by virus particles [8]. Thus, GP5 protein has important significance in pathogenicity, diagnosis, prevention and control of PRRSV [10].
In this study four major procedures: GP5 protein purification; immunization of inactivated PRRSV; fusion of spleen and SP/20 cells and screening by ELISA were carried out to identify potential mAbs for research and practical applications [9].Although BALB/c mice are not a natural host of PRRSV, they serve as a perfect bio-reactor to generate mAbs against all expressed structural and non-structural viral proteins. The neutralizing Ab titer of the immunized BALB/c mouse showed a high titer and the Abs produced from the recombinant protein immunized BALB/c should be similar to the live virus. Therefore, the mAbs generated here would be suitable for studies on viral relatedness and vaccine strain choice.
In this study, Two mAbs, 4B4 and 8C9, were isolated from the cell fusions. Using the PRRVS GP5 protein, prepared 4B4 mAbs were shown to have an excellent neutralizing activity against PRRVS, but 8C9 failed to show any neutralizing activity against PRRVS. One of the critical factors for the neutralizing activity of anti-PRRVS antibodies might be the high affinity binding between antagonists and PRRVS which is necessary to maintain a stable neutralizing complexes.













 DownLoad:
DownLoad: