-
White spot syndrome virus (WSSV) is one of the major and most serious pathogens of shrimp disease [2, 8, 11, 13]. The virulence of WSSV is very high, resulting in mortality rates from 90 to 100% within 3 to 7 days of infection and leading to complete devastation of the shrimp culture industry. WSSV belongs to a new virus family, Nimaviridae, under a new genus Whispovirus [7], which is an enveloped virus with a 305-kb double-stranded circular DNA genome [14].
Due to the lack of an adaptive immune mechanism in crustaceans, all efforts are directed towards the development of diagnostic tests for rapid and efficient detection of WSSV at early stages of the disease in order to minimize losses. Several techniques such as histological observation, in situ hybridization, PCR, western blot analysis or loop-mediated isothermal amplification (LAMP) have been developed [6, 9]. Some of them are laborious and time-consuming, while others have practical limitations to their wide spread application because of high cost and the need for special equipment and well-trained skilled personnel. Therefore, an antibody-based assay presents an attractive alternative, providing a simple and low-cost detection system with high specificity and optimal sensitivity for disease monitoring. Immunological diagnosis based on anti-WSSV immune serum as well as monoclonal antibodies (MAbs) have been used successfully to detect WSSV antigens [10, 12], but most of these MAbs were produced with purified WSSV or native envelop proteins of WSSV.
Since the purification of WSSV from infected shrimp is a laborious and time consuming task, we considered whether MAbs could be produced with recombinant envelope proteins from WSSV. A MAb specific to recombinant VP19 was developed to detect WSSV [1], but no MAb specific to recombinant VP28 was reported before. VP28 is one of the most abundant envelope proteins of WSSV [16] and is crucial for virus entry and vaccine-induced protection [5, 17]. Hence the present article reports the development of MAb against recombinant VP28 (rVP28) that can detect WSSV rapidly and sensitively at the early stages of WSSV infection.
HTML
-
The virus used in this study originated from WSSV-infected penaeid shrimp, Penaeus chinensis, from the East China Sea in 2001. Virus stocks were generated in crayfish Procambarus clarkia. Briefly, crayfish weighing approximately 20g were tested by PCR for the presence of WSSV to ensure that they were WSSV-free before the experiments and then injected intramuscularly with 100 μL of different virus dilutions in 330 mmol/L NaCl in the fourth or fifth abdominal segment using a 26-gauge needle. Dead crayfish were tested for the presence of WSSV by PCR and purified by differential centrifugation as we described previously [3] and examined by transmission electron microscopy (TEM, JEM-1200EX).
-
The DNA fragment encoding the entire VP28 ORF was amplified from genomic WSSV DNA by PCR. The primers were designed based on the published sequence of the WSSV genome [14] as follows: sense primer, 5'-CAGGATCCATGGATCTTTCTTTCACTC-3' (BamHI); anti-sense primer, 5'-GCAAGCTTTTAC TCGGTCTCAGTGC-3'(Hind Ⅲ). The amplified PCR product was ligated in pUCm-T vector (Promega) and sequenced (model 377, PE Applied Biosystems). The VP28 fragment was cut from pUCm-VP28 by digesting with BamHI/HindⅢ and ligated into pET-30a vector (Novagen). The construct was transferred into BL21 cells. The resulting Escherichia coli expression sample was analysed by SDS-PAGE and rVP28 fused with His6 tag was purified by Ni His-tag affinity chromatography (Qiagen).
-
Eight week old female Balb/c mice were used for immunization.Initially the mice were injected intraperitoneally with purified rVP28 protein (50 µg/mouse) and mixed with complete Freund's adjuvant. The mice were boosted three more times at 2 week intervals with the same quantity of antigen in incomplete Freund's adjuvant. One week after the fourth injection, mouse antiserum was collected and tested against lysates of E. coli containing either the pET-30a or pET-VP28 plasmid by Western blot. The best-performing mouse was subsequently boosted 3 days before hybridoma production.
Fusion was carried out according to Kohler and Milstein (1976) with the modifications described by Makesh [12]. A P3X myeloma cell line was used as the fusion partner. Two weeks after hypoxanthine aminopterin thymidine (HAT) (Gibco-BRL) selection, the hybridomas were screened for antibody. Positive cells were cloned by the limiting dilution method and stored at -80℃ in aliquots.
-
WSSV was purified by differential centrifugation as established before [3] and virions were mounted on carbon-stabilized nickel grids and blocked with 3% bovine serum albumin, and then incubated with diluted anti-rVP28 MAb. After incubation with gold-conjugated secondary antibody (Promega), the immuno-complexes formed were visualized with TEM.
-
Lysates of E.coli BL21 containing either pET-30a or pET-VP28 plasmid or gill homogenate samples from uninfected or WSSV-infected crayfish were applied to a prepared polyvinylidene difluoride membrane (PVDF, Promega) membrane and baked at room temperature for 60 min. The membrane was then blocked with 5% skim milk powder in phosphate buffered saline (PBS) for 45 min. The skim milk powder was removed by washing the membrane twice with Tris buffered saline containing 0.05% Tween-20 (TBST) and anti-rVP28 MAb was added and incubated over night. The membrane was washed with TBST and alkaline phosphatase-labeled goat-anti-mouse antibody (Promega) was added and incubated for 1 h at 37℃. The membranes were developed with BCIP/NBT color development substrate (Promega).
The analytical sensitivity of the test was estimated by five fold serial dilution of gill homogenate from WSSV-infected crayfish. Diluted homogenate was spotted onto PVDF membranes and processed for dot-blot analysis using the anti-rVP28 MAb and VP28-specific scFv antibody P108B4 (Provided by Dr. Heping Dai), which was used for positive control [15].
-
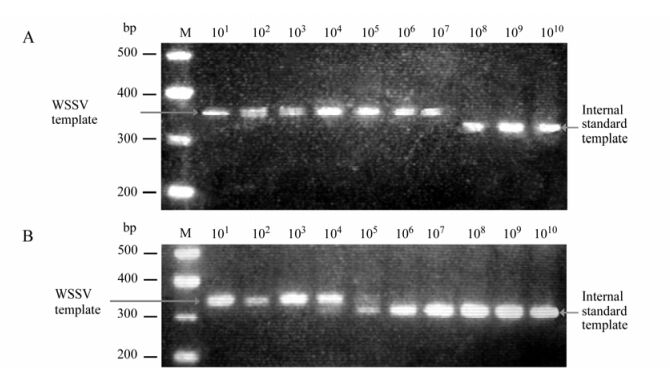
Using the method described previously [4], PCR was performed with two WSSV334F (5'-CTTTCACTC TTTCGGTCGTG-3') and WSSV334R (5'-TTCTGC CCCACAGTCACTTC-3') primers. For competitive PCR, DNA template (nucleic acids extracted from the gill homogenate used in the above dot-blot experiment) and equal volume of internal standard plasmid serially diluted in 10-folds were added to PCR reaction solution. PCR products were analyzed by electrophoresis on 2% agarose gels stained with ethidium bromide and visualized by ultraviolet transillumination.
Virus preparation
Cloning, expression, and purification of recombinant VP28
Production of anti-rVP28 monoclonal antibody
Immuno-electron microscopy
Dot-blot immunoassay
Competitive PCR to detect WSSV
-
The morphology of the negatively stained purified WSSV virions was rod-shaped to somewhat elliptical with an integral envelope (Fig. 1) as reported previously. Recombinant VP28 protein expressed in E.coli was examined by SDS-PAGE and Western blot analysis (Fig. 2). Purified rVP28 (Fig. 2, land 1) was used to inoculate mice. After hybridoma production, a total of 216 clones were obtained from the three fusions with a cloning efficiency of 42% of which 22 clones secreted antibodies that reacted with WSSV and not with crayfish tissue homogenate. Out of the 22 clones only 8 were stable on expansion and three clones secreted high titres of antibodies (as tested by indirect ELISA, data not shown). Of the three selected clones, the clone secreting the highest titre of antibody (anti-rVP28 MAb) was selected for bulk production of antibodies.
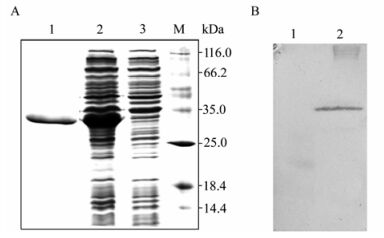
Figure 2. SDS-PAGE and Western blot analysis of expressed VP28. A: Proteins in the 15% gel were stained with Coomassie brilliant blue. Lane 1, Purified rVP28; 2, pET-VP28; 3, Empty pET-30a; M: Markers. B: Western blot with anti-His6 monoclonal antibody. Lane 1, Empty pET-30a; 2, pET-VP28.
Immuno-electron microscopy was employed to evaluate the possibility of detecting WSSV by anti-rVP28 MAb. The gold particles were distributed in the outer envelope of WSSV virions (Fig. 3A) and no gold particle was seen in the nucleocapsid (Fig. 3B). The results not only proved that VP28 is a virus envelope protein, but also showed that anti-rVP28 MAb could recognize native VP28 target epitopes of WSSV.
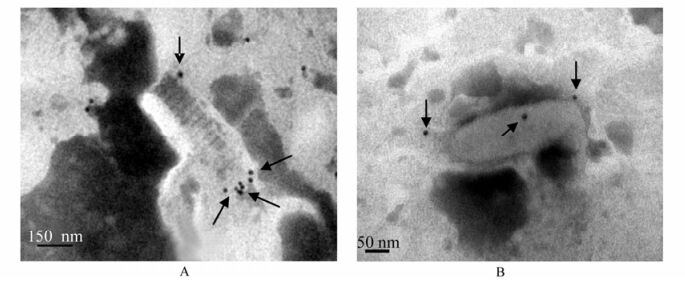
Figure 3. Localization of VP28 on the envelope of WSSV virions by immuno-electron microscopy. Anti-rVP28 MAb was used as first antibody and detected by Goat anti Mouse IgG labeled with colloid gold. The binding colloid gold particles are indicated with black arrows. A: WSSV with unpacking envelope and envelope is gathering at the end of the nucleoca psid. B: the WSSV with tightly packed envelope.
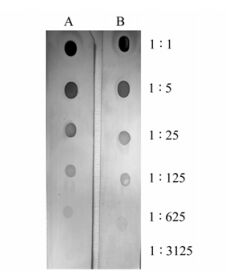
Anti-rVP28 MAb and scFv antibody P108B4 were used in dot blot assays for detection specificity. Anti-rVP28 MAb bound intensely to WSSV-infected crayfish homogenate as effectively as the positive control (P108B4) (Fig. 4). Two other major shrimp viruses, taura syndrome virus (TSV) and infectious hypodermal and hematopoietic necrosis virus (IHHNV), were also detected but no dot was seen (data not shown). To determine the ability of MAbs to detect WSSV infection in field samples, a gill homogenate sample of WSSV-infected crayfish was serially diluted with normal crayfish gill homogenate for dot-blot analysis. As shown in Fig. 5, the dilution of WSSV detection limit with anti-rVP28 MAb and scFv antibody P108B4 was approximately 1:625. Even though scFv antibody P108B4 seemed to demonstrate stronger immunohistochemical staining than that of anti-rVP28 MAb, they possessed of identical detection limits as measured by dot-blot assays. At the same time, competitive PCR was employed to analyze quantity of WSSV in the gill samples used in the dot-blot experiment above. Results showed that the viral level remained at 107 copies/mg tissue in the original homogenate and levels were reduced as expected when diluted (Fig. 6). At a dilution of 1: 625, which was the detection limit of the dot-blot assays, the viral level was 104 copies/mg tissue. In our previous study [4], there were approximately 104 virions per mg gill tissues at 12 h postinfection at a water temperature of 24℃. At this time, infected crayfish or shrimp seem healthy and have almost no symptoms at all. This suggests that dot-blot analysis used in combination with anti-rVP28 MAb can detect WSSV rapidly and sensitively at the early stages of WSSV infection.
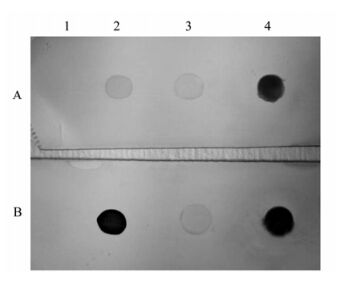
Figure 4. Dot-blot analysis of anti-rVP28 MAb. Lysates onto a PVDF membrane: 1, BL21 Escherichia coli transfected with pET-30a plasmid; 2, E. coli with pET-VP28 plasmid; 3, Gill homogenate from uninfected; 4, WSSV-infected crayfish spotted (5 μL / spot). Treated with scFv antibody P108B4 (A) or anti-rVP28 MAb (B).













 DownLoad:
DownLoad: