-
In 2009, humankind encountered its first pandemic of the 21st century in the form of a new variant of the influenza A (H1N1) pdm09 virus containing a triple reassortment of RNA segments from human, swine and avian influenza strains [3, 20]. Unlike the regular seasonal influenza virus, the emerging pandemic virus was notable for its rapid spread throughout the population [3]. According to the World Health Organization (WHO), as of June 25, 2010, the influenza A (H1N1) pdm09 pandemic had affected 214 countries and caused 18, 209 human deaths [17]. An accurate estimate of the total number of infected people could not be established because per WHO recommendations, swab screening for the influenza virus was canceled in July 2009. Such tests would have exceeded the capacity of laboratories even in most well-developed countries where the incidence rate was high.
Large-scale vaccination of the population is the most effective countermeasure in controlling pandemic outbreaks. However, due to significant genetic mutations in the pandemic influenza A (H1N1) pdm09 virus, seasonal vaccines against influenza failed to ensure appropriate cross protection [5, 12]. Therefore, many pharmaceutical companies throughout the world developed, conducted clinical trials, and licensed monovalent pandemic influenza vaccines based on actual recombinant vaccine strains. For example, in 2009, four brands of the pandemic influenza vaccine were ready for use in the European Union (EU): Celvapan by Baxter (Czech Republic/Austria), Pandemrix by GSK (Belgium), Focetria by Novartis (Italy), and Fluval P by Omninvest (Hungary). Production of all licensed pandemic vaccines was based on use of the original isolate of influenza virus A/California/7/2009 (H1N1) pdm09. These vaccines were found to be safe and highly immunogenic in humans [4], and per WHO recommendations, pandemic strain antigens of influenza virus A/California/7/2009 (H1N1) pdm09 have been included in seasonal trivalent vaccines since 2010.
While the development and introduction of effective vaccines is an important measure for suppressing influenza pandemics, the current production capacity cannot fully meet the world's demand for pandemic vaccines [6]. As part of a strategic plan to provide the population of Kazakhstan with sufficient stocks of vaccines against pandemic influenza, within the limits of the national scientific program for 2009-2011, the country has developed the first Kazakhstan egg-derived, inactivated, whole-virion adjuvanted vaccine Refluvac®against influenza strain A (H1N1) pdm09. The vaccine was developed by the Research Institute for Biological Safety Problems (RIBSP) using the recombinant influenza strain NIBRG-121xp, which was provided by the National Institute for Biological Standards and Control (NIBSC, U.K.). We show the safety of the developed preparation as indicated by pre-clinical trials conducted at the lead scientific research centers in Russia (The Research Institute of Influenza, Institute of Toxicology, Chemical and Pharmaceutical Academy) and Kazakhstan (The National Center for Expertise on Drugs) [9]. In this report, we present positive results for the immunogenicity and efficacy of the vaccine in ferrets, and recommend a dose of a vaccine for a phase I clinical trial.
HTML
-
This study was carried out in compliance with national and international laws and guidelines on laboratory animal handling. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Research Institute for Biological Safety Problems (Permit Number: 117).
-
Standard laboratory procedures for the production of the vaccine Refluvac®are based on the reassortment strain NIBRG-121xp (NIBSC code: 09/166) that was obtained by NIBSC (U.K.) through reverse genetic engineering from influenza strains A/California/7/2009 (H1N1) pdm09 and A/PR/8/34 (H1N1). This strain was provided to RIBSP by WHO for use in vaccine development. The vaccine virus was cultured in 10-to 11-day-old chicken embryos that came from facilities free from acute infectious diseases and that tested seronegative for the influenza A virus. The embryos were infected with a virus dose of 10 000 EID50 and cultured for 72 h at 37 ℃ and a relative humidity of 60%. Viruses were inactivated by incubation with formaldehyde (Sigma, Germany) at a final concentration 0.05% for 72 h at 4 ℃. Viruses were purified and concentrated by filtration through membrane filters (Millipore, U.S.A.) with a pore size of 0.45 μm, ultrafiltration/diafiltration using the Pellicon® cassette system (Pellicon 100 000 NMWL; Millipore, USA.), gel filtration with sepharose CL-6В (Sigma, Germany), sterile filtration through membrane filters (Millipore, USA.) with a pore size of 0.22 μm, and sorption of the purified virus concentrate on aluminum hydroxide (Alhydrogel "85", 2% solution, with an aluminum ion content of 10.0 mg/mL (Brenntag). The virus hemagglutinin concentration in the intermediate vaccine prior to addition of the sorbent agent was determined by single radial immunodiffusion [19] using monospecific antiserum and antigen provided by NIBSC (U.K.) as standards. Thimerosal was added as preservative in a vaccine at a final concentration of 100 μg/mL. Vaccine quality parameters were assessed according to the specifications set forth in the Pharmacopoeia Standard of Manufacturer.
To test the immunogenicity and protective level of vaccine, 3 samples were prepared by the vaccine, the composition of which is given in Table 1.

Table 1. Composition of vaccine samples
-
For each of the three test vaccine samples, 0.5 mL was injected intramuscularly into the hind legs of eight 6-to 7-month-old female ferrets (Biotest, Czech Republic). The vaccine was given either as a single dose or as two doses 14 days apart. Ferrets from the negative control group (n = 8) were similarly injected with 0.5 mL PBS containing 0.5 ± 0.1 mg of aluminum hydroxide (Al+3). The animals were distributed into groups by randomization. Lack of outward signs of the disease and homogeneity of groups by body weight (±20%) were used as randomization acceptance criteria.
To assess the protective effect of the vaccine, on the 14th day after a single or double vaccination, ferrets from the experimental and negative groups were sedated with ketamine (intramuscularly 20 mg/kg) and xylazine (intramuscularly 1 mg/kg) and infected intranasally with pandemic strain A/California/7/09 (H1N1) pdm09. This strain was provided by the Research Institute of Influenza (St. Petersburg, Russia) and given at a dosage of 106 EID50/0.5 mL (0.25 mL in each nasal duct). The infected animals were clinically observed (sneezing, dynamic activity, appetite, discharge from the eyes and nose, breathing, etc.) for 14 days and daily temperature and body weight measurements were taken.
On the 3rd, 5th, 7th and 14th day after infection, nasal lavage (from the nasal turbinate) was conducted in the vaccinated and negative control groups of ferrets, and the fluid obtained was stored in tubes with 1 mL of virus transport media (sterile solution of calf infusion broth, fraction V of bovine albumin, gentamicin sulphate, and Fungizone®). The selected lavage samples were kept at −70 ℃ for further titering in 10-day-old chicken embryos.
All tests with the pandemic virus took place in a Biosafety Level 3 environment.
-
On the 14th day after single or double vaccinations, blood samples were collected from the ferrets to be tested by the hemagglutination inhibition test [11]. To remove non-specific inhibitors, blood test samples were treated with the receptor-destroying enzyme from Vibrio cholerae (Denka Seiken Co. Ltd., Japan). Eight hemagglutinating units of the NIBRG-121xp virus were added to serial dilutions of the test serum in PBS, and the mixture was incubated at 37 ℃ for 30 min. A 0.5% suspension of rooster erythrocytes was added and sedimented, and the antibody titer was determined based on the highest serum dilution that suppressed virus hemagglutination. The detection limit of the HAIT was 10.
-
The infectious activity was identified by virus titering in 10-day-old chicken embryos using conventional methods. Ten-fold dilutions of the virus suspension in PBS (from 10-1 to 10-10) were prepared. Four chicken embryos were injected with 0.2 mL of each dilution in the allantoic cavity. The chicken embryos were cultivated for 3 days at 33℃ and a relative air humidity of 55%. Virus presence in chicken embryos after culturing was confirmed by hemagglutination reaction [18]. The virus titer was calculated by the method of Reed and Muench and was expressed in log10 EID50/0.2 mL [13].
-
The sample mean (Х), root-mean-square error (m) and confidence interval of mean values were determined. The reliability of differences between indicators (p < 0.05) was determined using Student's t-test.
Bioethics compliance
Vaccine preparation
Study of immunogenicity and protection conferred by the vaccine on ferrets
Hemagglutination Inhibition Test (HAIT)
Infectious activity of the virus
Statistical processing of experimental data
-
In order to establish preliminary recommendations for the use of the vaccine Refluvac®in humans (an immunization schedule), the clinical trial stage included an evaluation of the vaccine's immunogenicity in ferrets in relation to dosage and frequency of administration. Groups of eight ferrets were injected with vaccine specimens containing 3.75, 7.5 or 15.0 μg/dose of the virus hemagglutinin strain NIBRG-121xp. Inoculations were given as single doses or with a booster given 14 days later. On the 14th day after single or double vaccinations, ferrets from each group were bled for HAIR. A similar protocol was followed with a negative control group (n = 8) that was only injected with PBS and adjuvant. Vaccine immunogenicities between the experimental and control groups were evaluated by measuring the geometric mean titer (GMT) of antibodies (Fig. 1).
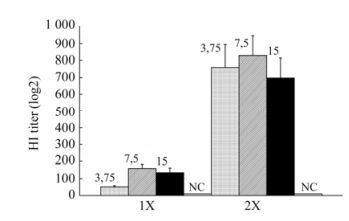
Figure 1. Immunogenicity of pandemic vaccine Refluvac®against influenza A (H1N1) pdm09 in ferrets depending on the dose and frequency of administration. Groups of ferrets (n = 8) were injected with vaccines having a hemagglutinin content of 3.75, 7.5 or 15.0 μg and 0.125, 0.25 or 0.5 mg of Al+3 per dose respectively. On the 14th day after single and double administrations, ferrets were bled for HAIR. Standard error of GMT antibodies is shown as the error bar. 1×, single vaccination; 2×, double vaccination 14 days apart; NC, negative control.
Irrespective of the antigen load and adjuvant (Al+3) content, all tested vaccine specimens were immunogenic in ferrets as both single and double vaccinations. Statistical analysis showed significant differences (P < 0.025) between antibody titers in single-vaccinated animals receiving 3.75 μg (HI titer, 51 ± 7) and animals receiving 7.5 μg (HI titer, 160 ± 23) of hemagglutinin per dose. Differences between the GMT of antibodies in ferrets injected with the vaccine having an antigen load of 7.5 μg (HI titer, 160 ± 23) and 15.0 μg (HI titer, 134 ± 28) of hemagglutinin per dose were insignificant (P > 0.2).
The boost-vaccination increased the GMT of antibodies in all experimental groups of ferrets. The antibody titer in animals on the 14th day after a second vaccination had increased 5.1–14.9 times (HI titer, from 697 ± 120 to 829 ± 117). No statistically significant differences (P > 0.2) between the GMT of antibodies in groups of ferrets injected with vaccines having 3.75, 7.5 or 15.0 μg HA/dose were found when the vaccine was given as two doses, 14 days apart.
Antibodies to A(H1N1) v in the negative control group ferrets injected with PBS were not detected.
-
The protective effect of the vaccine preparation against influenza infection was evaluated using ferrets (8 animals/group) that had been injected with vaccines containing 3.75, 7.5 or 15.0 μg HA/dose or with PBS alone. On the 14th day after a single or double vaccination, ferrets from the experimental and negative groups were infected with the pandemic influenza strain A/California/7/09 (H1N1) pdm09 as per the methods described in Materials and Methods.
During the 14-day clinical observation of the infected ferrets (Table 2), no clinical signs were found in any of the ferrets that had been vaccinated and none of the vaccinated ferrets had died. Dynamic activity, appetite, and social behavior of the ferrets in the vaccinated groups were similar to those in quarantine. The animals' fur was clean and shiny, without any hair loss spots.

Table 2. Clinical observation of ferrets from experimental (vaccinated) and control (PBS) groups infected with pandemic virus A/California/7/09 (H1N1) pdm09
The reference group of ferrets that had been injected once or twice with PBS and adjuvant began showing signs of influenza on the 3rd day after infection. On the 4th–7th day after infection, five or six ferrets showed sneezing, low dynamic activity, lack of appetite, and watery discharge from the nose and eyes that later became denser and formed a coating around the nostrils. The ferrets also were breathing heavily due to nasal obstruction. By the end of the observation period, the ferrets from the reference group had recovered.
-
An analysis of the body weight after the controlled infection (Fig. 2) showed that the group of ferrets that were injected once with the 3.75, 7.5 or 15 μg/dose vaccine showed minor weight loss (0.8–2.8%) a few days after infection. By the end of the 14-day observation period, all experimental groups had a positive body weight gain of 2–5.1%. No body weight loss was seen during the whole observation period in groups of revaccinated ferrets, irrespective of the antigen load of the vaccine used. The body weight growth in groups of ferrets double-injected with the 3.75, 7.5 or 15 μg/dose vaccine was 7%, 8.4% and 6.3%, respectively, on the 14th day after infection. Negative control groups of ferrets expressed clinical signs of the disease, including lack of appetite, and had significant body weight loss that peaked on the 6th day (15.5–15.7%). Some body weight growth was observed on the 14th day but the animals' weights remained about 10% lower than that before the experiment.
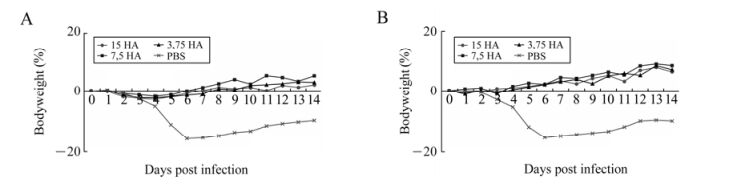
Figure 2. Changes in body weight of ferrets from the experimental and control groups after infection. Changes in the body weight of the group of ferrets (n = 8) that had been injected once (A) or twice (В) with pandemic vaccine with a hemagglutinin content of 3.75, 7.5 or 15 μg/dose. The observation period was 14 days. The animals' body weight is shown as the percentage relative to the starting weight before the experiment (0%).
-
The body temperatures of ferrets from all experimental groups after infection were within normal range during the whole observation period (the normal temperature range for ferrets is between 37.8 and 39.4 ℃) (Fig. 3). Higher temperature was only observed on the 4th day after infection in the group of ferrets single-injected with the vaccine with 3.75 μg HA/dose. The negative control groups of animals had higher body temperature on the first four days after infection. The highest body temperature in ferrets was recorded in the first three days (40.3 ± 0.1 ℃), and by the 6th day after infection, the animals' body temperatures returned to normal and stayed that way until the end of the experiment.
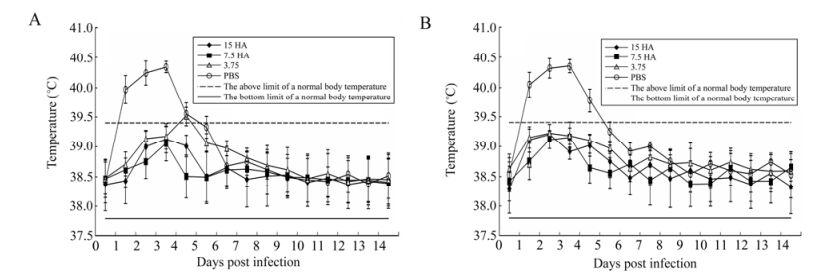
Figure 3. Changes in the body temperature of ferrets from the experimental and control groups after infection. Changes in body temperature of the group of ferrets (n = 8) given single injections (A) or two injections (В) of pandemic vaccine with a hemagglutinin content of 3.75, 7.5, and 15 μg/dose. The observation period was 14 days, and the standard deviations (SD) for mean values of body temperature in groups are provided as error bars.
-
An analysis of virus shedding in the upper airways of infected ferrets from experimental and reference groups (Table 3) showed that vaccination of animals, irrespective of frequency of administration and antigen load, significantly reduced virus shedding in nasal turbinates during the infection period as compared with animals from the PBS-injected control group. On the second day after infection, 3–5 animals in each group that had been singly injected with the vaccine showed virus shedding (titers ranged from 1.16 ± 0.38 to 2.25 ± 0.46 log10 EID50/0.2 mL) from their nasal turbinates. In the reference group, 7 animals shed viruses with high titers (4.67 ± 0.55 log10 EID50/0.2 mL). On the fifth day after infection, all the animals in the reference group were shedding the virus through their nasal turbinates, but the virus titers had decreased to 2.0 ± 0.45 log10 EID50/0.2 mL. On the seventh day after infection, no viral shedding was observed in any of the ferrets, from the experimental and control groups.

Table 3. Titer of A/California/7/09 (H1N1) pdm09 in nasal lavage fluid of ferrets from the experimental and control groups after infection
Irrespective of the antigen load, double-injected ferrets had very low virus shedding in the nasal turbinates on the second day after infection (titers were less than 1.37 ± 0.17 log10 EID50/0.2 mL). The number of animals shedding the virus was also low (1–2 animals per group). From the fifth day to the end of the experiment, no virus shedding from nasal turbinates was observed in animals from the experimental groups. The negative control group of infected ferrets (double-injected with PBS) had the same level of pandemic virus shedding as the animals receiving a single injection.
Vaccine immunogenicity in ferrets according to dose and frequency of administration
Protective effects of the vaccine in ferrets
Change in body weight of animals after the controlled infection
Change in body temperature of animals after the controlled infection
Virus shedding in the upper airways of infected ferrets
-
Development of an inactivated, whole-virion adjuvant (aluminum hydroxide) vaccine Refluvac®against pandemic influenza A (H1N1) pdm09 in Kazakhstan was based on reports [7, 10, 15, 16] that demonstrated that this vaccine type, as compared with split and subunit types, has a higher immunogenicity level both in laboratory animal models and humans during clinical trials. Moreover, the immunity conferred by inactivated whole-virion vaccines shows cross-protection, i.e. the vaccine is able to protect people even from genetic variants of the pandemic influenza virus [10, 16]. It is critical to note that the innocuity of whole-virion influenza vaccines is not significantly differrent from that of their split and subunit equivalents.
The vaccine Refluvac®production process described here is simpler, cheaper and more efficient than existing technologies, which is important for production of this vaccine in underdeveloped countries. The vaccine production process consists of the following core stages: accumulation of the vaccine virus in chicken embryos; inactivation of the viral biomass with formaldehyde; virus purification and concentration by membrane filtration and chromatography; and sorption of the treated virus concentrate on aluminum hydroxide. The fundamental difference between this method and other techniques is the virus treatment and concentration stage: instead of the zonal centrifugation methods currently in widespread use, it utilizes the relatively simple, cheap and efficient methods described in detail in Materials and Methods. The protocols described in this paper resulted in the production of highly purified virus (99.9% pure) in quantities suitable for manufacture of the inactivated influenza vaccine. It should be noted that we have carried out special experiments on the purification of viruses contained within the allantoic fluid before and after inactivation with formalin. Most manufacturers purify and concentrate the virus prior to inactivation. According to the results of our research, it is possible to inactivate the virus while it is still contained in the allantoic fluid and then to perform the purification and concentration of the viral antigen, as shown in the research of Lin et al. [7]. The advantage of the given purification method is the maximum removal of formalin, a carcinogenic substance, from the viral material.
As the literature suggests higher immunogenicity of influenza vaccines with adjuvants in comparison with equivalent preparations without adjuvants [4, 15], our vaccine composition included an optimized quantity (based on the sorption capacity) of aluminum hydroxide as an adjuvant.
The quality control results from three lots of vaccine Refluvac® (with a hemagglutinin content of 3.75 μg/dose) at intermediate stages as well-finished products established that the proposed technology is reproducible and results in a vaccine product that meets all requirements of the National and European pharmacopoeias (Table 4). During the pre-clinical testing of the vaccine described in our earlier paper [9], it was established that the vaccine, in terms of acute and subacute toxicity, allergenicity, and impact on the immune system, is safe and can be used for clinical trials. After the confirmation of vaccine safety in preclinical trials, we studied the immunogenicity and efficacy of the vaccine in ferrets, and recommend a dose of a vaccine Refluvac®for a phase I clinical trial.

Table 4. Quality control results for the three lots of vaccine Refluvac®
Analysis of the literature [1, 10, 14] with regard to the immunogenicity and protection conferred by inactivated, whole-virion influenza vaccines with adjuvants suggested that our preparation would be immunologically effective. As expected, inoculation of ferrets (n = 8) with our vaccine was immunologically effective both with single and double administrations and at all tested doses (Fig. 1). An increase in antibody titers, as measured by HAIR, was observed in ferrets that had been vaccinated. Even a single vaccination of ferrets with the vaccine having a hemagglutinin content of 3.75 μg/dose resulted in expressed immunity in the animals.
The true test of the efficacy of the vaccine in ferrets was in whether or not it would offer protection against influenza infection. Immunization of ferrets with the vaccine—irrespective of its hemagglutinin content and vaccination frequency—ensured 100% protection from clinical manifestation of the disease during a controlled infection with pandemic virus A/California/7/09 (H1N1) pdm09. The reference group of animals that did not receive the vaccine showed the characteristic symptoms of an influenza infection [8].
The body weight of the vaccinated ferrets after infection did not decrease during the 14-day observation period and the animals' body temperatures (except for ferrets single-injected with the vaccine at 3.75 μg HA/dose) were within the physiological range. The number of ferrets shedding the virus through their upper airways, as well as the amount of virus shed, after infection with pandemic virus was much lower in the vaccinated groups than in the reference group.
Our experimental data suggest that our egg-derived, inactivated, whole-virion adjuvanted vaccine Refluvac®against pandemic influenza A (H1N1) pdm09 with a hemagglutinin content of 3.75, 7.5 or 15 μg/dose is immunogenic and confers protection in ferret models with both single and double administrations. To conduct Phase I clinical trials, a single vaccination with a dose containing 3.75 or 7.5 μg hemagglutinin is suggested due to its adequate efficacy in our current work. In case of positive results from clinical tests of the vaccine (I-phase) with the chosen vaccination scheme, we will be able to increase a volume and to reduce terms of production at the expense of low content of hemagglutinin, that is extremely important during pandemic influenza period.













 DownLoad:
DownLoad: