-
Reactivation of hepatitis B is a well-recognized complication in hepatitis B virus (HBV) infected patients undergoing cancer chemotherapy [2]. HBV reactivation usually occurs with fulminant hepatitis and hepatic failure in patients with hematological malignancies who receive anthracycline, rituximab, and steroids treatment as anticancer therapy [4, 7, 9, 14]. The nucleoside analogue, lamivudine (LMV) can significantly decrease HBV DNA and serum transaminases levels, as well as restore tissue function in compensated chronic hepatitis B infection [1, 6]. The safety and efficacy of LMV treatment for patients with severe acute or fulminant hepatitis B has been extensively documented [5, 12, 13], and most studies consider that LMV treatment can improve the survival rate of severe acute or fulminant hepatitis B patients [10, 13]. Moreover, LMV and interferon as prophylactic antiviral agents can significantly reduce the incidence of HBV reactivation and the overall morbidity of cancer patients with HBV positive undergoing chemotherapy [3]. This strategy may, however, fail.
Here we report a surface antigen of an HBV (HBsAg) positive cancer patient who developed fulminant exacerbation of chronic hepatitis B despite the administration of antiviral therapy with LMV after cytotoxic chemotherapy. The sequence analysis of this antigen revealed multiple mutation sites in HBV genotype C.
HTML
-
A 62-year-old man with chronic hepatitis B infection was diagnosed with liver cirrhosis in 1995. Virologic markers were positive for HBsAg, anti-HBe and anti-HBc. Later the patient was found to have malignancy and received chemotherapy. In March, 2007, the liver specific enzymes were abnormal (110 U/L alanine aminotransferase (ALT), 67 U/L aspartate aminotransferase (AST)) (Fig. 1), suggesting the reactivation of hepatitis. Antiviral therapy with LMV 100 mg daily was started immediately. However, after one week's treatment, hepatitis still progressed. Screening for virus marker displayed the following pattern: HBsAg, anti-HBc (IgG) and anti-HBe were positive; anti-HBs, HBeAg and anti-HBc (IgM) were negative. Serum HBV DNA was detected and the viral load reached 4.6×106 copies/mL (Fig. 1), and ALT and AST rose to 215 U/L and 83 U/L, respectively. In April, 2007, ALT was 186 U/L and AST was 95 U/L. Thereafter, liver enzymes of the patient increased rapidly. After 17 days, ALT and AST were at 1587 U/L and 1968 U/L (Fig. 1), respectively. At the same time, jaundice began to occur (7.1 μmol/L total bilirubin (TB)) (Fig. 1). Sonography result showed acute liver damage. To recover liver function, Konakion was administered intravenously for 3 days, and Ursofalk 250 mg was added to the daily 100 mg of LMV. The levels of ALT and AST then showed a slight reduction (1407U/L ALT, 1748U/L AST), whereas the level of TB still rose continuously (9.3 μmol/L and 11 μmol/L, respectively in two routine screenings in April) (Fig. 1). Surprisingly, anti-HBs (435 IU/L) and anti-HBc (IgM) became positive (Fig. 1). Serum HBV DNA load reached 1.1×107 copies/mL (Fig. 1). Thereafter, aminotransferase levels were mildly reduced (Fig. 1). However, at the end of April, a rebound of aminotransferase level occurred (1435 U/L ALT, 1745 U/L AST). TB stayed at a high level of about 15 μmol/L during April (Fig. 1). Finally, the patient died due to hepatic failure in May, 2007.
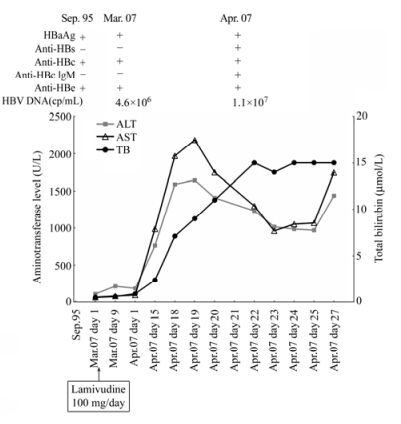
Figure 1. Patient's clinical course and viral status. HBV DNA, hepatitis B virus DNA; anti-HBc, anti-hepatitis B core antigen antibody; IgM, immunoglobulin M; anti-HBs, anti-hepatitis B surface antigen antibody; anti-HBe, anti-hepatitis B e antigen antibody; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin.
Before seroconversion of anti-HBs, the sequence analysis of the small HBsAg protein revealed HBV genotype C (HBsAg subtype adw) with several remarkable amino acid substitutions (F8L, S34L, F41S, G44V, F93C, V96G, L110I, T131N, C149Y and F161Y), mostly outside the "a" determinant (Fig. 2). However, after seroconversion of anti-HBs, a new substitution L109Q occurred, while the C149Y substitution disappeared (Fig. 2) [11].
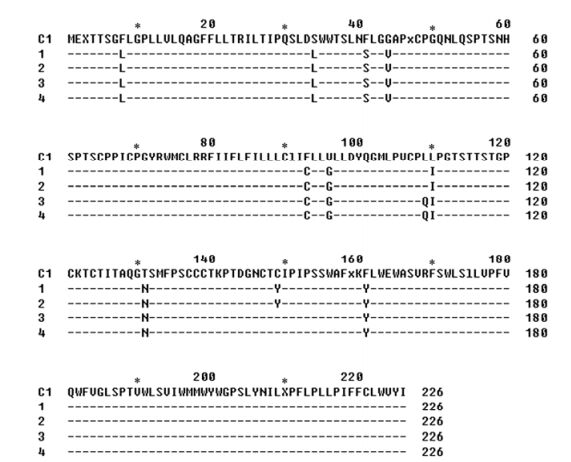
Figure 2. Amino acid sequences of small HBsAg protein before and after seroconversion of anti-HBs. The sequences were aligned to the deduced amino acid sequence of genotype C1 [15]. Sequences 1 and 2 were derived from the sera of patient before seroconversion of anti-HBs; Sequences 3 and 4 were derived from the sera of patient after seroconversion of anti-HBs.
-
LMV treatment is recommended for patients with severe acute or fulminant hepatitis B caused by genotype B and D, to prevent HBV reactivation and hepatic failure [10, 13]. However, the efficacy of LMV treatment for patients with fulminant hepatitis B caused by genotype C remains unclear. Our case suggested that HBV genotype C patients might develop more severe exacerbations than genotype B or D patients and be less susceptible to LMV. Thus, the importance of genotype in the acute exacerbation of HBV leading to fatal liver failure should be further considered.
The patient's serum marker and DNA load showed an unusual pattern. Anti-HBs turned from negative to positive in less than one month, whereas HBsAg was constantly positive. The anti-HBs antibodies were detected using a commercial enzyme immunoassay AUSAB kit (Abbott Laboratory, USA), which detects the specific antibody to wtHBsAg. Therefore, the co-existence of anti-HBs antibodies and HBsAg suggested the occurrence of immune escape mutants. Furthermore, HBV DNA load continuously increased, and DNA sequencing revealed the presence of amino acid substitutions in HBsAg. All these data suggested a HBV reactivation caused by mutants, which could escape the host immune response and dominate the acute HBV infection that leads to the acute progress. This suggests that HBV immune-escaped mutants can be essential for HBV reactivation related-fulminant hepatitis.
The mutation of P gene corresponding to mutations within S region due to the LMV treatment was also analyzed, I16T, T38A, M129V, L132M and Y148H within RT region of P gene being found. Notably, LMV-resistant YMDD mutants were not found. The significance of these mutations for the HBV replication is not yet clear and will need further investigation.
Although LMV prophylaxis is recommended for patients infected with hepatitis B virus (HBV) undergoing chemotherapy for malignant disease, HBV reactivation sometimes occurs during or after LMV administration. The mechanism is not clear. Recent literature has reported that patients with high viremia, rituximab treatment, and distant metastasis are at high risk of prophylactic failure [4, 15]. In addition, preexistence of cirrhosis and higher baseline bilirubin level are considered to be significantly independent predictors of fulminating progression to hepatic failure [4]. Therefore, the optimal strategy for antiviral prophylaxis in HBsAg-positive patients with oncologic and hematologic malignancies undergoing chemotherapy should be further established.













 DownLoad:
DownLoad: