HTML
-
We report a case of HBV reactivation in an anti-HBs positive, anti-HBc positive non-Hodgkin's lymphoma patient. Hepatitis B virus (HBV) reactivation is a well-recognized complication of patients undergoing chemotherapy or immunosuppressive therapy for lymphomas. The presence of antibodies to the hepatitis B surface antigen (anti-HBs) has been identified to be a factor preventing HBV reactivation in patients with occult HBV infection receiving chemotherapy. In this paper, we present a non-Hodgkin Lymphoma patient who, before immunosuppressive therapy, displayed positive anti-HBs and positive antibodies to hepatitis B core antigens (anti-HBc), as markers of resolved HBV infection, and developed hepatitis B surface antigen (HBsAg) and high viraemia with an HBV escape mutant after rituximab-based administration. The sequencing data revealed HBV genotype D with two known escape mutations, P120S, and S154P, and three mutations, Y134K, I150T and T189I, which have not been found in the usual escape settings. Increasingly aggressive chemotherapy or immunosuppressive treatment could induce the reactivation of HBV and the outgrowth of HBsAg escape mutants in anti-HB positive patients. Early screening for HBV status and prompt administration of antiviral agents are crucial for prevention of HBV reactivation and fatal liver failure in anti-HBs positive patients.
It is well known that HBV can cause persistent or self limiting infections, depending on the immune response of the host. Neutralizing antibody to hepatitis B surface antigens (HBsAg), anti-HBs, is present in resolved HBV infection, whereas presence of non-protective antibodies to hepatitis B core antigen (anti-HBc) indicates chronic or resolved HBV infection. However, even in resolved cases, HBV DNA may persist in the liver, although its replication is suppressed by the immune reaction of the host(Alexopoulou A, et al., 2006; Awerkiew S, et al., 2007). Thus a previous HBV infection may be reactivated during drastic immunosuppressive therapy or chemotherapy and may lead to fatal hepatitis disease(Awerkiew S, et al., 2007; Iannitto E, et al., 2005; Onozawa M, et al., 2005; Sarrecchia C, et al., 2005). On the other hand, the presence of anti-HBs has been identified to be a factor that can prevent HBV reactivation in patients with occult HBV infection receiving chemotherapy(Lee I C, et al., 2010). In previous reports, HBV reactivations occured in the majority of HBsAg -positive patients(Yamagata M, et al., 2007). In contrast, HBV reactivation in patients with anti-HBs is extremely rare. Here, we report a non-Hodgkin lymphoma patient positive for anti-HBs and anti-HBc prior to immunosuppressive therapy, who developed HBsAg and high viraemia with an HBV escape mutant.
A 43-year-old man was diagnosed with non-Hodgkin lymphoma in 2005. Chemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) was started immediately and completed within four months, for a total of six cycles. After extended field radiotherapy, a complete remission was achieved. Subsequently, a relapse was treated with three cycles of R-DHAP (rituximab, dexamethasone, cisplatinum, AraC, mebthera) chemotherapy one year later for two months. Then one cycle of high dose CMV schema (Cyclophosphamid, VP16, Melphalan, Dexamethosan) with Gemcitabine (1500 mg) was performed twice, in the following two months, respectively. Finally, the patient received additional two cycles of stem cell therapy in parallel to the last cycle of chemotherapy.
Before R-DHAP therapy, the patient was HBsAg negative, anti-HBc and anti-HB (18 IU/L) positive, although there were no records on vaccination against hepatitis B. Thus the patient had a resolved HBV infection previously. However unexpectedly, four months after CMV schema and stem cell therapy, a strongly positive HBsAg reaction ( > 250 IU/L) was detected by using a microparticle enzyme immune assay (AxSym, Abbott, Germany) accompanied by disappearance of anti-HBs. HBV DNA tests revealed a high viral load ( > 1.8×107 copies/mL) in the following two months, respectively. Antiviral therapy with lamivudine 100 mg daily was started immediately. Thereafter viral load decreased and HBV DNA could not be detected one month after the start with lamivudine treatment. The patient is currently alive, and the tumor is in stable disease status. Liver function tests have been regularly monitored. The serum aminotransferase levels were normal throughout the courses of chemotherapy. After virus rebound, the liver-specific enzymes were still normal or only slightly elevated (Fig. 1).
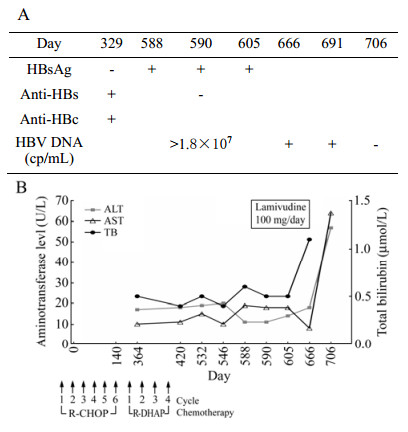
Figure 1. Patient's clinical course and viral status. (A) Hepatitis B serological and virological test results. (B) Serum aminotransferase (ALT, AST) and total bilirubin (TB) levels during and after rituximab-based chemotherapy. R-CHOP=rituximab, cyclophos?phamide, pegylated liposomal doxorubicin, vincristine, and prednisone. R-DHAP = rituximab, dexamethasone, cisplatinum, AraC and mebthera.
The coding sequence of the small HBsAg protein was determined after PCR amplification by using the primers 5'-GCACACGGAATTCCGAGGACTGGGGACCCTG-3' (nt 131-148) and 5'-GACACCAAGCTTGGTTAGGGTTT AAATGTATACC-3' (nt 845-825) (Reference sequence AY220698) as described previously (Zhang J M, et al., 2007). TI150T, S154P and T189I outside the "a" determinant.
HBV reactivation is a well-recognized complication of patients undergoing chemotherapy or immunosuppressive therapy for lymphomas(Tsutsumi Y, et al., 2004). Several risk factors of HBV reactivation have been identified, including male gender, young age, pre-existing liver disease, absence of anti-HBs, and HBV DNA level among HBsAg-positive and HBsAg-negative patients(Ferreira R, et al., 2012; Lee I C, et al., 2010; Sera T, et al., 2006). The risk is further increased in the absence of anti-HBs during rituximab plus steroids and rituximab combination chemotherapy(Ferreira R, et al., 2012; Lee I C, et al., 2010).
The association between rituximab treatment duration and HBV reactivation remains unclear. In our case, HBV reactivation developed after a total of 10 cycles of rituximab-based chemotherapy administration in 14 months. Previous reports have shown that in HBsAg-negative, anti-HBc-positive lymphoma patients receiving rituximab administration, the time of HBV reactivation ranged from the 2nd to the 8th course of anticancer therapy(Dahlen D D, et al., 2003; Lee I C, et al., 2010; Yamagata M, et al., 2007; Yeo W, et al., 2009). Moreover, in our anti-HBs-positive patient, HBV reactivation developed after 6 months of the last rituximab administration. A recent report stated that in HBsAg-negative, anti-HBs-positive lymphoma patients, the time of HBV reactivation ranged from 1st cycle of rituximab-based chemotherapy to 32 months after stopping rituximab combination chemotherapy(Ferreira R, et al., 2012).
Generally, anti-HBs-positivity has been considered to present few copies of HBV, and HBV rarely is reactivated under such circumstances(Sera T, et al., 2006). Although the presence of anti-HBs has been identified to be a factor to prevent HBV reactivation in patients with occult HBV infection receiving chemotherapy(Lee I C, et al., 2010), few cases of HBV reactivation in anti-HBs positive lymphoma patients have been described in previous reports(Matsue K, et al., 2010; Nainan O V, et al., 2002). However, the mechanism of HBV reactivation in lymphoma patients in the presence of anti-HBs has not been well clarified. One possible explanation is that increasingly aggressive chemotherapy or immunosuppressive treatment induced the reactivation of HBV and th e outgrowth of escape mutants in anti-HBs positive patients, and reactivated escape mutants were selected, as has been observed previously(Alexopoulou A, et al., 2006; Awerkiew S, et al., 2007; Westhoff T H, et al., 2003). Thus our findings further suggest that the HBsAg mutants could be the prominent strains in anti-HBs positive patients in rituximab based HBV reactivation. Obviously, the escape mutations didn't impair the viability of the virus because it reached very high viremia and HBs antigenemia.
In addition, the patient described here had mutations, additional to the two known escape mutations P120S and S154P, including Y134K, I150T and T189I, which have not been found in the usual escape settings(Carman W F, et al., 1995; Nainan O V, et al., 2002; Protzer-Knolle U, et al., 1998). Moreover, the previously described P120S mutation has been identified in one HBsAg-vaccinated child of an HBsAg-positive mother(Mele A, et al., 2001) and S154Phas previously been confirmed to have a reduced sensitivity in immunoassays(Moerman B, et al., 2004). Thus, vaccine induced anti-HBs were unable to protect against the well-known mutant efficiently. Furthermore it is uncertain whether the vaccine could provide protection against escape mutants with more mutations. Prot ection of immunocompromised patients by currently available HBIG preparations seems to be ineffective against HBV with escape mutations(Awerkiew S, et al., 2007).
Use of antiviral agent as prophylaxis of HBV in HBsAg positive patients undergoing cytotoxic chemotherapy is becoming a standard strategy(Fukushima N, et al., 2009). This strategy may, however, fail. We previously reported a surface antigen of an HBV (HBsAg) positive cancer patient who developed fulminant exacerbation of chronic hepatitis B despite the administration of antiviral therapy with LMV after cytotoxic chemotherapy(Wu C, et al., 2012). Moreover, for occult HBV carrier patients, the clinical benefits of routine prophylactic antiviral treatment have not been established. Our case indicates that close monitoring of serum aminotransferase levels, early recognition of HBV reactivation, and prompt use of antiviral agents resulted in suppression of HBV DNA levels, normalization of liver function, and avoidance of hepatic failure.













 DownLoad:
DownLoad: