HTML
-
The occurrence of prion diseases, a group of neurodegenerative diseases that includes bovine spongiform encephalopathy (BSE), Kuru, Gerstmann-Sträussler-Scheinker disease (GSS), Creutzfeldt-Jakob Disease (CJD) and Fatal Familial Insomnia (FFI), is rare, amounting to about 1-2 cases per million people per year, amongst which 85% are sporadic, 15% are inherited and less than 1% are infectious. The cellular prion protein (PrPC) is a cell surface glycosylphosphatidylinositol (GPI)-anchored glycoprotein that is expressed in many tissues besides the central nervous system (Harris D A, 1999; Prusiner S B, et al., 1998), including in the islets of the pancreas-especially in the β cells (Strom A, et al., 2011; Strom A, et al., 2007), although one report has suggested that it is expressed in the α cells (Amselgruber W M, et al., 2006). Li et al. (2009) reported that PrP expressed in some pancreatic cancer cell lines existed as pro-PrP and was a biomarker for poor prognostic for pancreatic cancer patients. Importantly, no PrP was detected in the pancreatic ductal cells of the non-cancerous tissue. Thus, expression of PrP in cancer cells may contribute to the tumorigenesis of pancreatic cancers. To elucidate the role played by PrP during the transition from normal ductal cells to cancer cells, it is important to determine whether PrP is detectable in normal ductal cells. PrPC is thought to be the essential substrate for scrapie prion protein (PrPSc)-caused transmissible spongiform encephalitis (TSE), which is apparently the result of the conformational change of the α-helix-rich PrPC into the β-sheet-rich PrPSc. The mechanism of this conformational change remains the core question in this particular field.
Because PrPSc tends to aggregate and is difficult to dissolve in most buffers, routine techniques such as X-ray crystallography and nuclear magnetic resonance (NMR) are difficult to apply in structural studies of PrPSc. Monoclonal antibodies (Mabs) with defined epitopes have been shown to be able to detect protein conformational changes (Han J, et al., 2011; Saunders D N, et al., 1998; Yin S, et al., 2007). In the present study, therefore, we characterize a panel of monoclonal antibodies that have not been extensively studied previously; and we explore whether the human pancreatic ductal epithelial cell (HPDC) expresses PrPC. The results demonstrate that HPDC does not express PrPC, which is consistent with the implications of our previous work (Li C, et al., 2009).
-
BxPC3 (a human pancreatic ductal adenocarcinoma cell line) and HPDC were purchased from American Type Culture Collection (ATCC). BxPC3 cells were cultured in RPMI1640 supplemented with 1.5 g/L sodium bicarbonate, 10% fetal bovine serum (FBS)(Gibco, Grand Island, NY, USA), 1% sodium pyruvate, 1 mmol/L HEPES, 4.5 g/L glucose, 100 U/mL of penicillin and 100 U/mL streptomycin. HPDC was cultured in Dulbecco's Modified Eagle Medium (DMEM) (Sigma, St. Louis, MO, USA) supplemented with 2 mmol/L L-glutamine, 1.5 g/L sodium bicarbonate, 25% Medium M3 Base (Incell Corp., San Antonio, TX, USA), 5% FBS (Gibco), 10 ng/mL human recombinant epidermal growth factor (EGF), 5.5 mmol/L D-glucose, 750 ng/mL puromycin, 100 U/mL of penicillin and 100 U/mL streptomycin. PrP-knockout cells derived from brain neural cells of PrP-knockout mice, WV and WVT cells were obtained from Professor Man-Sun Sy at Case Western Reserve University (Cleveland, Ohio, USA). PrP-knockout cells were cultured in DMEM (Gibco) supplemented with 3.17 g/L sodium bicarbonate, 10% FBS (Gibco), 3 g/L HEPES, 100 U/mL of penicillin and streptomycin. WV and WVT cells were human neuroblastoma cell lines transfected with full-length human PrP and truncated (90-253) human PrP, respectively. These cells were cultured in RPMI1640 (Gibco) supplemented with 1.5 g/L sodium bicarbonate, 10% FBS (Gibco), 1% sodium pyruvate, 1 mmol/L HEPES, 4.5 g/L glucose, and 100 U/mL of penicillin and 100 U/mL streptomycin.
Anti-PrP Mabs (1A1, 1A6, 1G6, 2A10, 2C2, 2C8, 2C8.4, 2C9.31, 2D4.3, 2F8, 4A1, 4E9, 4C4, 4E5, 4H2, 5B2, 5E2, 6B4, 6B6, 6D4, 6D11, 6G4, 6H3, 7A9, 7A12, 7A12.1, 7B8, 7C11, 7E6, 7F11, 8B4, 8C6, 8F9.1, 8G12, 8H4, 9H7, 10G4, 11G5, 12A3, 12A4, 12G10, 12H7, 14G7, 14C9, and DG1) and biotinylated 8H4 were kindly provided by Professor Man-Sun Sy. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgGspecific antibody was purchased from AntGene Biotech (Wuhan, China). Mouse anti-actin Mab was purchased from Tianjin Sungene Biotech, Tianjin, China (Cat. No. KM9001). Mouse IgG1 isotype control and HRPstreptavidin were purchased from Biolegend (San Diego, CA, USA). 4', 6-Diamidine-2'-phenylindole dihydrochloride (DAPI) was purchased from Roche (Mannheim, Germany). Alexa Fluor® 488 dye-linked goat anti-mouse secondary antibody was purchased from Invitrogen Corporation (Carlsbad, CA, USA). All reagents purchased from commercial sources were used according to the suppliers' recommendations.
-
96-well plates (Costa) were coated with affinity-purified Mabs (20 ng/well) at 4 ℃ overnight, and were then rinsed well with PBST (phosphate-buffered saline (PBS) with 0.05% Tween-20) six times. The coated plates were blocked with 3% bovine serum albumin (BSA) in PBS at room temperature (RT) for 3 h. 100 μL of diluted BxPC3 lysate or PrP-knockout cell lysate was added to each well and incubated at RT for 2 h; the wells were then washed with PBST six times, prior to the addition of biotinylated 8H4 (20 ng/well) and incubation at RT for 1 h. After six washes with PBST, HRP-streptavidin was added to the wells and the plates were incubated at RT for 30 min. The plates were then washed six times with PBST. 100 μL of 3, 3', 5, 5'-Tetramethylbenzidine (TMB) substrate (Sigma, Cat. No. 860336) was then dispensed to each well and incubation at RT was continued for an additional 20 min, followed by the addition of 50 μL of 2 mol/L H2SO4 to each well to stop the reaction. The absorbance was determined at 450 nm and 570 nm on a Kinetic Micro-plate Reader (Perkin Elmer, Cat. No. 2300, Akron, Ohio, USA). The results were the average of duplicates and all experiments were repeated twice with comparable results.
-
For immunofluorescence staining, BxPC3 and PrPknockout cells were cultured in 24-well plates overnight. Cells were rinsed 3 times with ice-cold PBS and fixed in 4% paraformaldehyde for 15 min at RT. The plate was then rinsed three times with PBST (PBS with 0.1% Tween-20) and blocked with 10% goat serum plus 1% BSA in PBST, with incubation at RT for 2 h. Anti-PrP Mabs or mouse IgG1 isotype control were then incubated with the cells for 1 h with gentle shaking. After six washes with PBST, bound antibody was detected with an Alexa Fluor® 488 dye-conjugated goat anti-mouse IgG-specific antibody (Invitrogen). Nuclei were revealed with DAPI. Samples were analyzed using an immunofluorescent microscope (Zeiss, Jena, Germany). All experiments were repeated twice, with comparable results.
-
To determine whether the anti-PrP Mabs could be used for flow cytometry, BxPC3 cells were seeded into 6-well plates 12 h before use. The cells were then treated with 0.25% trypsin/EDTA. Anti-PrP Mabs or mouse IgG1 isotype control (10 ng/μL) were added to the cell suspensions at 4 ℃ and incubated for 1 h. Bound antibody was detected using Alexa Fluor® 488 dye-conjugated goat anti-mouse IgG-specific antibody (Invitrogen) and analyzed in a BD FACSTM C6 flow cytometer (San Jose, CA, USA). The experiments were repeated twice with comparable results.
-
Cell lysates were prepared in lysis buffer containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerol phosphate, 1 mmol/L Na3VO4, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). EDTA-free protease inhibitor cocktail and PMSF (1 mmol/L) was added just before making the lysate. Samples were resolved using 10% SDSPAGE and immunoblotted with anti-PrP Mabs; bound antibodies were detected with HRP-labeled goat antimouse IgG antibody. To confirm that PrP was present in the mouse brain lysate, the membrane was stripped using RestoreTM Plus Western Blot Stripping Buffer (Cat. No. 46430, Thermo Scientific, Waltham, MA, USA). After stripping, Mab 8H4 was applied to detect the same blots. Actin was used as the loading control.
-
Total RNA was isolated using a RNApure Tissue Kit (Cat. No. CW0584, CWBiotech, Beijing, China). One microgram of total RNA was used to synthesize first-strand complementary DNA (cDNA) using the PrimeScriptTM RT reagent kit with gDNA Eraser (Cat. No. DRR047A, Takara Bio, Shiga, Japan). Quantitative PCR amplification was carried out with primer sets (Forward-GTGACTATGAGGACCGTTACTATC; Reverse-TGACCGTGTGCTGCTTGA) derived from PRNP by using iQTM SYBR Green Supermix (Product No. 170-8882AP, Bio-Rad, Hercules, CA, USA) on a Bio-Rad ConnectTM real-time PCR instrument (CFX ConnectTM Optics Module). Each reaction was run in triplicate and contained 1 μL of cDNA tem plate in a final reaction volume of 20 μL. Melting curves were performed to ensure that only a single product was amplified. The relative expression of PrP was normalized to the expression level of β-actin. Data shown represent the meanstandard error of the mean from three triplicates.
-
Cell lines were cultured in poly-D-lysine-coated glass-bottomed petri dishes (Product No. P35GC-1.0-14-C, MatTek Corporation, Ashland, MA, USA) overnight. Antibodies specific for PrP (10 μg/mL) were incubated with cells for 1 h at RT. Bound antibodies were detected using Alexa Fluor® 488 dye-conjugated goat anti-mouse IgG-specific antibody (Invitrogen). Nuclei were counter-stained with DAPI and observed using an inverted confocal microscope (Perkin Elmer).
Cell lines, Mabs, and reagents
Characterization of Mabs with sandwich ELISA
Immunofluorescence staining
Flow cytometry analysis
Immunoblotting
Quantitative real-time polymerase chain reaction (PCR) analysis
Confocal microscopic imaging
-
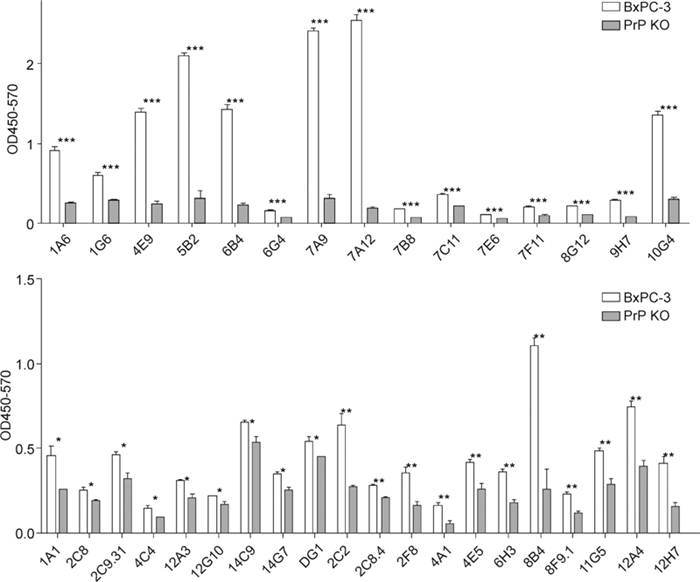
Firstly, we characterized the panel of monoclonal antibodies with ELISA to ensure that they were specific for PrPC. An ELISA plate was coated with 41 different Mabs raised against synthetic peptides of PrP. PrPknockout mouse cell lysate or wild-type mouse brain lysate was then loaded for 1 h. To detect captured PrP, biotinylated 8H4 was then incubated with the lysate and detected with HRP-conjugated strep tavidin. The majority of the antibody pairs reacted well with the BxPC3 cell lysate but not with the PrP-knockout cell lysate; 15 of them gave a high signal/background ratio (P < 0.001, and OD values for the knockout cell lysate were lower than 0.2) (Figure 1). These results therefore implied that in the ELISA reaction most of this panel of Mabs were specific for PrP.
-
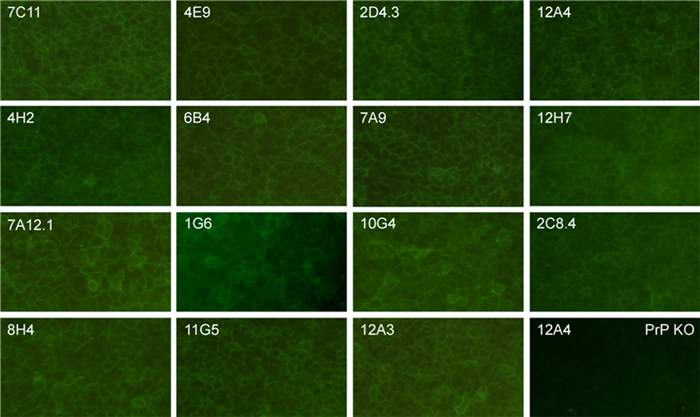
In order to determine whether the Mabs could be used for immunofluorescence staining, we stained BxPC3 cells, a pancreatic adenocarcinoma cell line expressing PrP. PrP-knockout cells were used as a negative control. We also included a mouse IgG1 isotype control in this study. Fifteen out of the 41 antibodies tested showed good membrane staining to BxPC3 cells, but none of them reacted with PrP-knockout cells. Isotype control antibody did not react with BxPC3 cells (Figure 2). It was therefore demonstrated that some of the Mabs could be used for immunofluorescence staining of PrP.
-
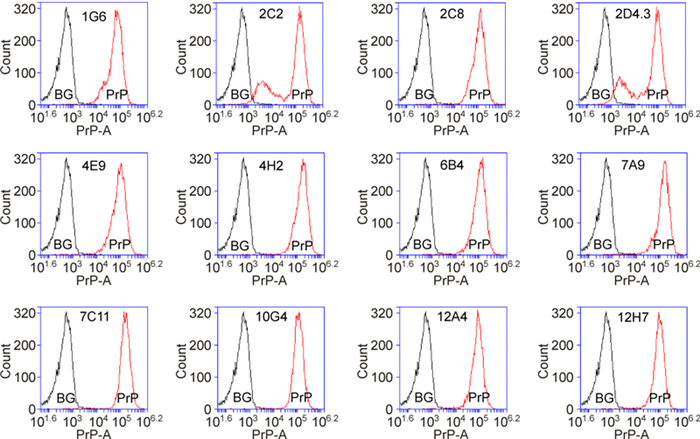
Flow cytometry is a widely used technique for immunofluorescence labeling of proteins. It is known that not all antibodies suitable for use in immunofluoresence staining can be used successfully in flow cytometry. Of the 41 antibodies tested, 13 Mabs specifically recognized cell surface PrP, as compared to isotype control (Figure 3).
-
Immunoblotting is widely used to show the relative amounts and apparent molecular weights of proteins. Two Mabs, 8H4 and 8B4, have already been extensively characterized for this purpose. Here, we showed that five other Mabs could be used to detect PrP by immunoblotting, although none was as sensitive as 8H4 (Figure 4).
-
Finally, we investigated whether or not HPDC expressed PrPC. Using immunohistochemical staining, we have shown previously that human normal ductal cells do not express detectable PrP by (Li C, et al., 2009). PrP mRNA was not detectable using quantitative PCR (Figure 5A). Consistent with this result, no PrP staining was observed in HPDC cells, whereas PrP was clearly detectable in WV cells, a neuroblastoma cell line transfected with PrP (Figure 5B). Furthermore, immunoblotting using Mabs 8H4, 4H2, 7C11 demonstrated the presence of PrP in mouse brain lysate but not in HPDC and PrPknockout cells (Figure 5C), suggesting that normal pancreatic ductal cells in pancreas tissue probably do not express PrP, in line with our previous observations.
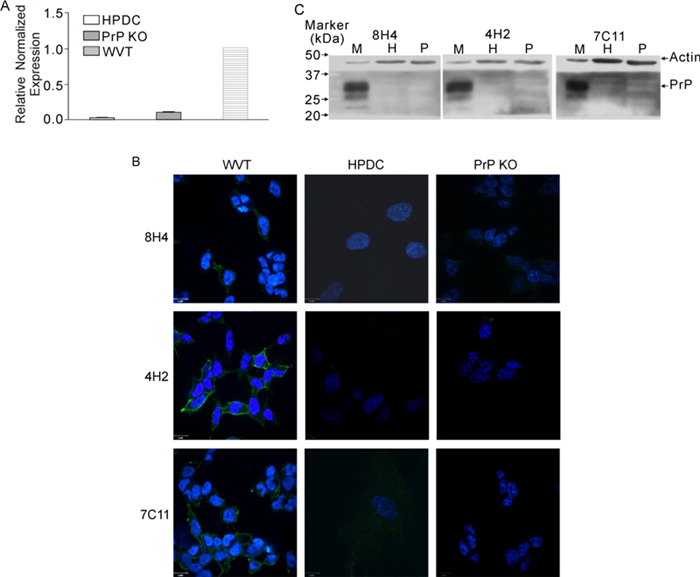
Figure 5. Absence of expression of PrP in HPDC cell line (PrP KO = PrP-knockout). (A) Quantitative PCR showing that the PRNP mRNA level is very low in HPDC. (B) Confocal microscopic images showing that WVT cells express PrP on the cell surface. HPDC and PrP-knockout mouse neuron cells do not express PrP. Original magnification, ×600. (C) Immunoblots showing that PrP from mouse brain has a MW of 22-31kDa, whereas there is no expression of PrP in HPDC and PrP-knockout cells. M, mouse brain; H, HPDC; P, PrP-knockout cell.
In conclusion, several Mabs raised to PrP could be used for ELISA, flow cytometry, immunofluorescence staining, and immunoblotting (Table 1). Under our test conditions, HPDC did not express PrP.
Anti-PrP Mabs 1A6 1G6 2C2 2C8 2C8.4 2D4.3 2F8 4A1 4E9 4H2 5B2 6B4 6G4 6H3 7A9 ELISA √ √ √ √ √ √ √ √ √ √ √ IFA √ √ √ √ √ √ √ FCM √ √ √ √ √ √ √ √ WB √ √ Anti-PrP Mabs 7A12 7B8 7C11 7E6 7F11 8B4 8F9.1 8G12 8H4 9H7 10G4 11G5 12A3 12A4 12H7 ELISA √ √ √ √ √ √ √ √ √ √ √ √ √ IFA √ √ √ √ √ √ √ √ FCM √ √ √ √ WB √ √ √ ? √ √ IFA: Immunofluorescent Assay; FCM: Flow Cytometry; WB: Western Blotting Table 1. Summary of the applications of the anti-PrP Mabs
The majority of the Mabs were specific for PrP in ELISA assay
Some of the selected Mabs could be used for immunofluorescence staining of PrP
Thirteen selected Mabs could detect cell surface PrP
Five further Mabs could be used to detect PrP by immunoblotting
No expression of PrP in human normal pancreatic ductal epithelial cells
-
No mad cow disease has ever been observed in China; however, CJD, GSS, and FFI have been reported and associated with different mutation frequency (Chen C, et al., 2011; Chen C, et al., 2013; Shi Q, et al., 2012; Shi Q, et al., 2013). The frequency of occurrence of TSE is comparable to that seen in other parts of the world, as are the symptoms and the disease duration (Chen C, et al., 2013; Gao C, et al., 2011). Although many cellular phenotypes have been associated with PrPC, none of this seems to be consistent with the fact that PrPC knockout mice develop apparently normally. The functions assigned to PrPC therefore warrant further investigation; hence, in order to study them, a panel of monoclonal antibodies was characterized in this work for different potential applications, including ELISA, immunofluorescence staining, flow cytometry, and western blotting. We found that 15 out of the 41 antibodies tested could be used for ELISA, 15 could be used for immunofluorescence staining and 13 could be used for flow cytometry; however, only 5 antibodies besides 8H4 and 8B4 could be used for immunoblotting (Table 1). Previously, some of the anti-PrP specific Mabs had been tested with paraffin-imbedded prostate and oral squamous cell carcinoma (OSCC) samples and it had been shown that, in addition to 8H4, two of the Mabs, 4H2 and 2D4.3, could be used successfully for immunohistochemical staining (Zhang J, et al., 2013).
Differing opinions have been expressed as to which islet cells express PrP (Amselgruber W M, et al., 2006; Strom A, et al., 2011; Strom A, et al., 2007). Interestingly, the expression of PrPC seems to affect blood glucose metabolism (Strom A, et al., 2011); compared to C57BL/6 control mice, male Zrch I PrPC -knockout mice showed significantly higher blood glucose concentrations following intraperitoneal glucose injection. It is tempting to hypothesize that PrPC may actually participate in the regulation of blood sugar homeostasis, bearing in mind that PrPC plays a role in glucose metabolism through the Fyn-HIF-2α-Glut1 pathway in a colon cancer cell line (Li Q Q, et al., 2011). In addition, vCJD-infected Macaca fascicularis normally develop signs of type Ⅱ diabetes, showing significant weight gain in comparison to non-BSE-infected control monkeys (Strom A, et al., 2013). In the mouse model of prion infection, different strains of PrPSc (139H or HY strain) may cause different body weight responses to infection (Bailey J D, et al., 2008; Carp R I, et al., 1989), suggesting that PrP may play a role in blood glucose homeostasis. After all, type Ⅱ diabetes may be the result of protein aggregation; and this may imply a role for PrP in the pathogenesis of type Ⅱ diabetes.
The observation that no PrPC was detected in HPDC is consistent with findings that we and others have reported previously (Amselgruber W M, et al., 2006; Li C, et al., 2009; Strom A, et al., 2007). However, around 50%–70% of PDAC tissues express PrP; and PrP-positive patients have poor prognosis, suggesting that PrP contributes to PDAC tumorigenesis. It is worth noting that PrP levels have been shown to be up-regulated in many different types of cancers, within which the normal tissues do not express PrP, or express it only at low levels (Yang X, et al., 2014). Thus it is important to identify the factors that control the expression of PrP and to elucidate the role played by PrP in the pathogenesis of diseases other than prion diseases.
-
We thank the National Natural Sciences Foundation of China (81172376, 31270209), the 100 talent-program of the Chinese Academy of Sciences and the State Key Laboratory of Virology for financial support.
-
All the authors declare that they have no competing interest. This article does not contain any studies with human or animal subjects performed by the any of the authors.
-
LY, YZ, and CL designed the experiment. LY, YZ, LH performed the experiments. CL, MS, and YZ wrote the paper.














 DownLoad:
DownLoad: