HTML
-
Enterovirus 71(EV71) belongs to the family Picornaviridae and remains a major pathogen causing Hand-and-foot mouth disease(HFMD) in children(McMinn et al., 2001; Pallansch and Roos, 2001). Since firstly isolated and identified in California in 1969(Chen et al., 2013), EV71 and its epidemic have frequently appeared in Asian countries including China, Singapore, Malaysia, and Japan over the past two decades(Girn et al., 2002; Halstead and O'Rourke, 1977; Chua and Kasri, 2011). The newest case control study shown several factors were involved in the HFMD acquisition and progress(Liu et al., 2014). The disease progress could be divided into four stages: first, HFMD and herpangina; second, central nervous system involvement; third, cardiopulmonary involvement; fourth, long-term sequelae. Most EV71 infections remain at stage one, and only very few proceed further to the most severe condition under the certain host condition or immune background.
Accumulating literatures reported that immunization-induced IgG against EV71 structure protein especially VP1 significantly correlates with the protection from EV71 lethal challenge. For example, Chung et al. demonstrated that immunization with virus-like particles of EV71 elicited potent immune responses and protected mice against lethal challenge(Chung et al., 2008). A phase Ⅲ EV71 clinical trial in China showed inactivated alum-adjuvanted EV71 vaccine provided above 90% efficacy(Zhu et al., 2013). It displayed the structure protein-induced high titer of IgG significantly correlated with the protection from EV71 infection.
However, whether asymptomatic infection-induced EV71 specific IgG in non-vaccinated children could provide protection from viral infection is not known. Many studies have verified the existence of antibodies to the enteroviruses in children due to the previously asymptomatic or unrecognized EV71 infection(Bell and McCartney, 1984; Li et al., 2013). A clinical observation between 1998 and 2008 found that some children frequently suffered from EV71 infection. Furthermore, children may be repeatedly infected by different members in enterovirus and get even worsen disease to next stage in viral infection(Wang et al., 2012). This suggested that the preexisting antibody induced in previous infection in children could not provide protection but might enhance EV71-related disease acquisition and progress.
In the present study, we detected the mucosal antibody responses against EV71 and the RNA copy number of EV71 from healthy children and young outpatients suffering the EV71 infection. We observed that preexisting antibody IgG against EV71 in local mucosal surfaces correlated with following viral infection. Our result suggested a mucosal antibody dependent enhancement(ADE) of EV71 infection in vivo.
-
The children were suspected to be HFMD, which clinically characterized by a brief, generally mild, febrile illness with papulovesicular rash on the palms and soles, and multiple oral ulcers. Pharyngo-laryngeal mucosal specimen from clinically suspected EV71-infected patient was collected and analyzed as follow.
Throat swabs were placed in 5 mL viral transport medium(Earle's balanced salt solution, 0.2% sodium bicarbonate, 0.5% bovine serum albumin, 200 µg of Vancomycin per liter, 18 µg of Amikacin per liter, 160 U of Nystatin per liter) in screw cap tubes and were transferred to the biosafety level 3(BSL-3) laboratory. After freezing and thawing, 5 mL of the throat wash was centrifuged at 1, 500 × g for 15 min to separate the supernatant from the mucous-cell pellet. Four milliliters of the supernatant were collected as the throat wash supernatant, aliquotted into four 1 mL tubes and stored at -80 ℃ until use. The samples were subjected to viral RNA extraction and EV71 antibody detection.
-
A commercially available EV71 diagnostic kit(Da An Gene Co., Ltd, Guangzhou, China) was used to detect EV71 in throat swabs sample. The detection method is based on a one-step real-time reverse transcription-polymerase chain reaction assay, and the sensitivity of the kit is 1 × 102 PFU/mL. A specimen is considered positive for EV71(EV71+) if the amplification curve crosses the threshold line within 36 cycles. RNA copy was calculated based on the standard curve. And those with EV71+ were randomly recruited in this investigation.
-
The purified protein or inactivated virus(VP2, VP1, 3D, or EV71)(5 μg/mL) in coating buffer(40 mmol/L Na2CO3, 60 mmol/L NaHCO3, pH 9.6) were respectively adsorbed to the surface of 96-well flexible microplates(Greiner Bio-one, Frickenhausen, Germany) at 4 ℃ over night. After discarding coating buffer, the swab transport medium supernatants were respectively incubated in the protein coated 96-well microplates for 1 h at 37 ℃. After washing 5 times with PBS-T, the plates were incubated for 45 min at room temperature with alkaline phosphatase-conjugated anti-human IgG antibody. After washing with PBS-T seven times, immunoreactivity was visualized by means of a pNPP phosphatase substrate system(KPL, Gaithersburg, MD) and the optical density values(OD405) were measured using an ELISA plate reader(Thermo Labsystems, MA). Pharyngo-laryngeal mucosal specimen giving OD405 values greater than the mean OD405 plus two standard deviations for healthy children controls were considered antibody response positive.
-
All data analysis was performed with a Spearman nonparametric test with GraphPad Prism software version 5.0.
Sample processing
Identification of EV71 in clinical pharyngolaryngeal specimen
ELISA
Statistical analyses
-
Twenty-nine throat swab samples from healthy children were collected under the permission of their parents or guardians in 2010. All the specimens collected from healthy children in this study were confirmed as negative for EV71 infection(EV71-) and had no clinical EV71-infection hospital record(detailed information not shown).
The time of samples collection span three years from 2010 to 2012. One hundred throat swab specimens were enrolled from young outpatients, which had been diagnosed clinically as suspected EV71 infection by physician and then further confirmed by molecular assay realtime PCR(EV71+). Eighty-six among one hundred patients were under 5 years old. Numbers of male and female patients are totally 69 and 31 respectively. Ninetysix patients went to a hospital within 3 days once clinical symptoms appeared. The detailed patient demographic information is summarized in Table 1.

Table 1. Characteristics of 100 children with clinically confirmed EV71 infection
-
In order to identify the possibility of existence of EV71-specific antibody IgG in clinically confirmed spec-imen, we examined them by ELISA coating VP1, VP2, 3D as well as purified inactivated EV71 virus particle. The specimens were four fold diluted and determined the IgG response for VP1, VP2, 3D and EV71. Value of OD405 by ELISA was used to reflect the level of IgG antibody. And for the same clinical sample, different IgG antibody profiles appeared when detected with distinct proteins or virus. Basically, antibody in most specimens could not be detected when thirty-two or more fold diluted, which therefore mean a relatively low-level of IgG titer for EV71 in mucosal specimen.
To discover the possible relationship of IgG titers between VP1, VP2, 3D as well as purified EV71 for each sample, we analysed the correlation of the IgG titers detected by different coating proteins by a Spearman linear regression. Resultantly, IgG response against any coating protein(VP1, VP2, 3D or EV71) significantly correlated with that of one another(p < 0.0001)(Figure 1). The results released that low-level of mucosal IgG antibody against EV71 broadly existed in children population, and IgG response against viral capsid protein significantly correlate with that against non-structure protein, e.g. 3D.
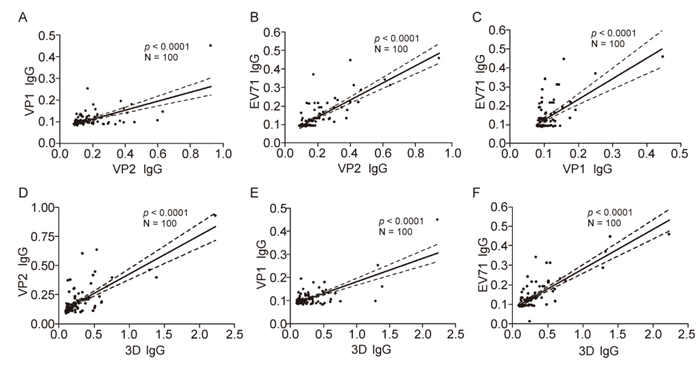
Figure 1. Relationships between IgG titers against different components of EV71 in the specimens with viral infection. The specimens were four fold diluted and determined the IgG response for VP1, VP2, 3D and EV71 by ELISA. Value of OD405 by ELISA was used to reflect the level of IgG antibody. And then the correlations of the IgG titers detected by different coating proteins were determined by a Spearman linear regression. The IgG titer of VP2 significantly correlated with that of VP1 (A) and EV71 (B) (p < 0.0001). The IgG titer of VP1 significantly correlated with that of EV71 (C) (p < 0.0001). The IgG titer of 3D significantly correlated with that of VP2 (D), VP1 (E) as well as EV71 (F) (p < 0.0001).
-
To assess the EV71-specific IgG profile in virus infected clinical sample, we first determined the cut-off value of IgG response against VP2, VP1, 3D, or EV71 in healthy control by ELISA. Throat swab specimens from twenty-nine healthy children were collected and measured for VP2 and 3D responses, in which RNA copy number was under 100 copies of virus/mL by using realtime PCR. Therefore, virus-or protein-specific IgG were reported as absorbance at 405 nm, and values exceeding [Mean(seronegative control)+ 2 standard deviations] were considered positive. Based on this formula, the cutoff values of IgG VP2, 3D, VP1 and EV71 were calculated to be 0.113, 0.142, 0.094, and 0.092 respectively(Figure 2A–D).
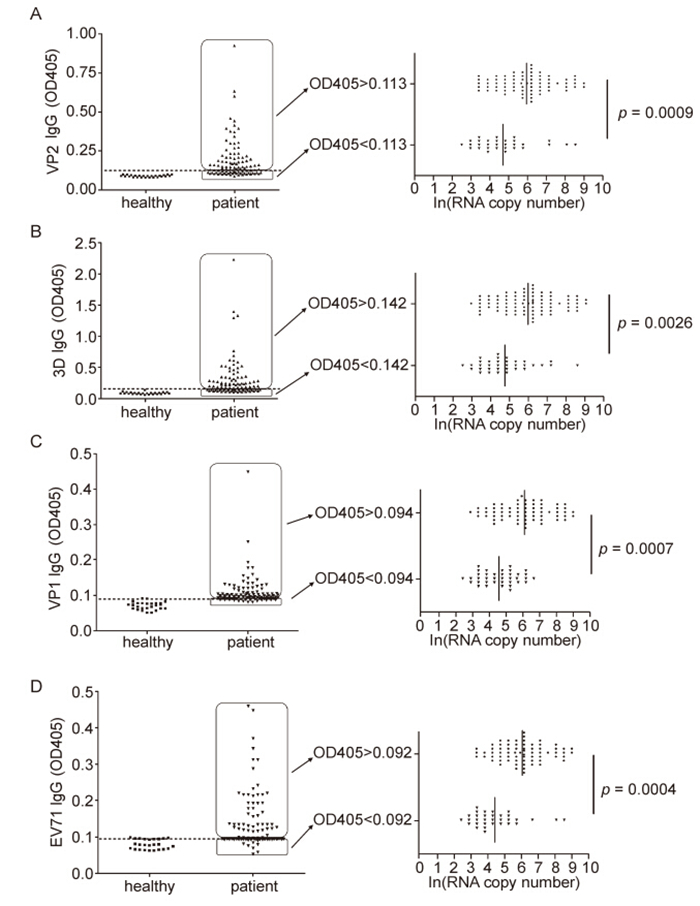
Figure 2. Relationships between IgG antibody titers and RNA copy numbers in EV71-infected clinical samples. IgG responses for VP2 and 3D of throat swab specimens from healthy children and EV71-infected patients by ELISA. Pharyngo-laryngeal mucosal specimen giving OD405 values greater than the mean OD405 plus two standard deviations for healthy children controls were considered antibody response positive. The cut-off value for VP2 IgG is 0.113 (discontinuity line). The mean RNA copy number of VP2 IgG positive group is strikingly higher than that of VP2 IgG negative group (p = 0.0009) (A). The cut-off value for 3D IgG is calculated 0.142 (discontinuity line), and the mean RNA copy number in 3D IgG positive group is prominently higher than that of 3D IgG negative (p = 0.0026) (B). Likewise, VP1 and EV71 antibody positive samples present significant EV71 RNA genome number than that of IgG negative samples (p = 0.0007 and p = 0.0004) (C, D).
Combing the information above mentioned that 96% patients went to a hospital within a few days once clinical symptoms appeared, we speculated that the occurrence of detected low-level of antibody must be induced due to the former EV71 infection rather than the ongoing sickness. In order to determine whether the preexisting viral antibody IgG correlate with the viral infection in vivo, we here divided the whole infected samples into two parts based on the antibody response against EV71 VP2: first group with IgG OD405 value < 0.113(without former VP2 IgG) and second group with OD405 value ≥ 0.113(with former VP2 IgG)(Figure 2A). The mean RNA copy number of VP2 IgG positive group is strikingly higher than that of VP2 IgG negative group(p = 0.0009)(Figure 2A). Meanwhile, the OD405 value under 0.142 was considered 3D IgG response negative, thus thirty specimens were negative(without preexisting 3D IgG) and seventy specimens were positive(with preexisting 3D IgG). Likewise, the mean RNA copy number in 3D IgG positive group is prominently higher than that of 3D IgG negative(p = 0.0026)(Figure 2B). Furthermore, VP1 and EV71 antibody positive samples present significant EV71 RNA genome number than that of IgG negative samples(p = 0.0007 and p = 0.0004). It therefore indicates that the children population with mucosal preexsiting EV71 IgG were prone to be infected.
-
In order to further evaluate the effect of preexsiting antibody on the EV71 infection and replication in vivo, we further analysed the relationship of EV71-specific antibody titer and viral copy number in each sample by a Spearman linear regression. Subsequently, the mean RNA copy number strikingly correlated with the VP2-IgG titer(p = 0.0101)(Figure 3A). Likewise, the mean RNA copy number prominently associated with 3D-IgG(p = 0.0042), VP1-IgG(p = 0.0189) and EV71-IgG(p = 0.0029) titer(Figure 3B–D). However, the difference of protein contents in each sample were not significant, which excluded the possibility that samples containing high amount of total material(cells, secretions) contain automatically higher amounts of virus and higher amounts of antibodies(data not shown). Overall, it further elucidated that the viral acquisition and replication increased accompanying the raise of mucosal preexsiting IgG response against EV71 structure and non-structure proteins, which suggested a former viral IgG mediated enhancement of viral infection in vivo.

Figure 3. Correlation between IgG titer and RNA copy number in infected specimens. The mean RNA copy number strikingly increases accompanied with the elevation of VP2-specific IgG titer (p = 0.0101) (A). Likewise, the mean RNA copy number strikingly increases accompanied with the elevation of 3D-specific (p = 0.0042) (B), VP1-specific (p = 0.0189) (C) and EV71-specific (p = 0.0029) (D) IgG titer. Each correlation was analyzed using a Spearman nonparametric test with GraphPad Prism software version 5.0.
Patient demographic data and diagnoses
Relationships between antibody response against viral structure and non-structure protein in infected specimens
Relationships between antibody response and RNA copy number in infected specimens
Correlation between IgG titer and RNA copy number in infected specimens
-
Since no commercial EV71 vaccine applicable and inoculated in children till now in China, and the samples were all collected from confirmed acute infected children, the detected EV71-specific IgG in pharyngo-laryngeal in this investigation therefore must be induced due to former EV71 infection rather than the ongoing sickness of acute infection when diagnosed. The results showed that preexisting antibodies against EV71 in local mucosal surfaces correlated with following viral infection, which suggesting an antibody dependent enhancement(ADE) of viral infection.
The phenomenon of ADE has been documented for many viruses, including dengue virus, respiratory syncytial virus, human immunodeficiency virus, and Ebola virus(Girn et al., 2002; Halstead and O'Rourke, 1977; Hober et al., 2001). ADE was also observed in many members of the Picornaviridae family including poliovirus and enterovirus such as EV71, coxsackievirus B, which were identified for targeting macrophage and monocyte in vitro(Tirado and Yoon, 2003; Wang et al., 2010). It was demonstrated that a reduction of maternal antibodies to the sub-neutralizing level within 1 year of age may be associated with the ADE of the infection(Wang et al., 2012). Han et al. coincidently demonstrated that the presence of sub-neutralizing antibody was able to increase mortality of EV71 infection in a suckling mouse model(Han et al., 2011). Moreover, EV71-neutralizing titer at about 1 : 512 dilution inhibited EV71 infection, whereas sera diluted to sub-neutralizing levels(5-to 0.5-fold) enhanced EV71 infectivity on THP-1 cells(Wang et al., 2010). However, the antibody responses against EV71 in mucosal surface and the relationship between the mucosal preexisting antibody against EV71 and possible secondary EV71 infection have not been investigated and remain elusive. We provided clinical evidence for mucosal EV71-specific antibody dependent enhancement of infection. In light of the human IgG subclasses(IgG1, IgG2 and IgG3) which manage distinct functions in vivo, Cao et al. have already studied the roles of each IgG subclass on neutralization and enhancement of EV71 infection, and found that the neutralizing activity of human IVIG is mainly mediated by IgG1 subclass and to less extent by IgG2 subclass, and IgG3 fraction did not have neutralizing activity but enhanced EV71 infection in vitro. Therefore, novel vaccine should be designed to induce the production of antibodies with strong neutralizing but weaker ADE activities.
Native infection result and animal immunization model showed that antigen specific IgG and IgA could be successfully induced, and appeared in animal mucosal tissue, such as nose lumen and genital lumen, via transcytosis by FcRn and pIgR(Bomsel et al., 2011; Li et al., 2011; Sun et al., 2012). The antigen specific antibody titer in mucosa is usually about 100-1000 fold lower than that in serum(Sun et al., 2012). During infection, EV71, like most viruses, enters the body through mucosal surfaces, and spreads to surrounding cells and tissues(Zhou et al., 2011). Nevertheless, it has not been addressed that the relatively low-level of capsid antibody of EV71, which preexisted in human mucosal microenvironment, would increase or inhibit the susceptibility to viral secondary infection. Accumulating evidences verified ADE of viral infection in vitro(Han et al., 2011; Tirado and Yoon, 2003) and in mouse model(Chen et al., 2013), accordingly our data also show the possibility that the low-level immune response enhanced viral infections in human mucosa.
High-level of system antibody response and mucosal antibody response were considered dominant for antiviral in adaptive immune response. EV71 vaccine in phase Ⅲ clinical trial achieved sufficient protective efficacy by inducing strong antibody response. However, considerable literatures and our data show that only low-level antibody response might mediate ADE of viral infection. Therefore, inactivated alum-adjuvanted EV71 vaccine immunization conferred over 90% efficacy which likely avoid the risk of antibody dependent enhancement of viral infection.
Many enteroviruses with different serotypes circulated throughout China annually during the past decade. Phylogenetic studies based on sequence alignment of the viral VP1 gene sequence have identified four genotypes(A, B, C and D) and numerous subtypes(e.g. B0-5, C1-5)(Ho et al., 1999). The VP1 was also considered the most important capsid protein during virus binding and entry(Yamayoshi et al., 2009; Nishimura et al., 2009), antibody for which was involved in the ADE of viral infection in vitro(Wang et al., 2010). Because of the VP1 sequence homology and similarity of viral infection procedure among member of family Picornaviridae, we speculated the antibody of EV71 clinically could enhance the CA16 or other virus in family Picornaviridae infection. In addition, nearly half of individuals infected with enterovirus suffered an asymptomatic infection, during which antibody including antigen specific IgG would still arised(Li et al., 2013), which could be another potential threat for broader ADE of viral infection.
Taken together, the high titer of IgG would neutralize or exclude virus from establishing infection, however preexisting low-level of IgG could facilitate the EV71 infection in mucosa and initiate exacerbation of patients. Therefore, these could be involved in EV71 vaccine design: 1) shorten the time span of antigen-specific antibody generation via enhancing the antigenicity of vaccine or adding immune adjuvant; 2) change alternative antigen as vaccine target void the potential ADE of virus infection, such as 3D, 3C.
-
This work was financially supported by the National Natural Science Foundation of China(Grant 81202381), the National Basic Research Program of China(973 Program)(Grant 2012CB518904). The authors also thank Dr. Wang and Dr. Gong from Wuhan Institute of Virology, CAS, China and Dr. Guo from Wuhan University for providing proteins 3D, VP2 and VP1.
-
The authors declared that they have no conflict of interest. The whole study was approved by the ethics committee of the Wuhan Institute of Virology(WIV), Chinese Academy of Sciences(CAS), China(No. WIVH09201301).
-
YL and HY designed the experiments. JX, YL, XX, JY, and HY carried out the experiments. YL and HY analyzed the data. YL and HY wrote the paper. All authors read and approved the final manuscript.













 DownLoad:
DownLoad: