HTML
-
Hand, foot and mouth disease (HFMD) is an infectious disease that has shown outbreaks in the Asia-Pacifc region in recent years, especially in mainland China (Ho et al., 1999; Fujimoto et al., 2002; Singh et al., 2002; Baek et al., 2009; Zhang et al., 2009; Chatproedprai et al., 2010). HFMD often occurs in children under the age of fve, causing mouth pain, loss of appetite, fever, and sores or ulcers in patients' hands, feet and mouths; it can cause neurological complications in some cases (Lee et al., 2009). Severe progression of HFMD can lead to death (Chonmaitree et al., 1981). HFMD is caused by 20 types of enteroviruses; enterovirus 71 (EV71) is the most common pathogen (Lee et al., 2009; Wong et al., 2010). EV71 belongs to the enterovirus group of the family Picornaviridae (Wong et al., 2010).
No effective therapeutic approaches or vaccines against HFMD are currently available. Live virus (Chang et al., 2011), inactivated virus (Chang et al., 2010), and recombinant VP1 protein (Wu et al., 2001; Chen et al., 2006; Sivasamugham et al., 2006; Chen et al., 2008; Li et al., 2009; Zhou et al., 2015) have been used to produce anti-EV71 antibodies. EV71 virus-like particles (VLP) are hollow viral particles that contain only the capsid proteins (VP0, VP1, and VP3) but lack viral nucleic acids, and which cannot replicate autonomously (Liu et al., 2011). VLPs are a new type of immunogen that can be used to develop antiviral antibodies (Roldao et al., 2010; Li et al., 2013); they possess complete epitopes, but are much safer than inactivated virus or viral proteins alone.
In this study, highly purifed EV71-VLPs produced in yeast were used to immunize mice to generate hybridomas for screening anti-EV71 antibodies. Two hybridomas, which produced antibodies that could effectively bind to EV71 were identifed. Furthermore, the antiviral capability of those two monoclonal neutralizing antibodies was evaluated.
-
The EV71-VLPs were produced in yeast and were provided by Shanghai Zerun Biotech Co., Ltd (Shanghai, China). The female BALB/c mice (aged 6–8 weeks) used in the immunization experiments were purchased from the Hubei Provincial Center for Disease Control and Prevention. One group of three mice was immunized subcutaneously with 20 μg of EV71-VLPs mixed with an equal volume of complete or incomplete Freund's adjuvant (for primary and booster injections, respectively) at weeks 0, 2, and 4. Blood samples from each mouse were collected from the tail artery at week 6 and their capacity to bind EV71 was determined through ELISA. The mouse with the highest titer was boosted with a double dose at week 6. Splenocytes were isolated from this mouse and fused with SP2/0 myeloma using PEG solution to generate hybridomas. After two weeks, hybridoma cells that could produce EV71-specific antibodies were screened by ELISA. Briefly, 20 ng of inactivated EV71 virus was coated on 96-well ELISA plates and incubated overnight at 4 ℃. Then the plates were washed with PBST three times, incubated with 100 μL of PBST containing 5% non-fat milk for 1 h, washed with PBST three times, incubated with 100 μL of hybridoma culture supernatant for 1 h, washed with PBST three times, incubated with 100 μL of anti-mouse IgG secondary antibodies conjugated with horseradish peroxidase for 1 h, washed with PBST three times, and incubated with 100 μL of chemiluminescent substrate. After color development, the reaction was stopped by addition of 100 μL of 2 mmol/L H2SO4 and the absorbance was measured at 450 nm.
-
Human rhabdomyosarcoma (RD) cells were cultured in DMEM (Dulbecco Modifed Eagle Medium) supplemented with 100 mL/L fetal bovine serum (FBS, Gibco BRL), 100 μg/mL of penicillin, and 100 μg/mL of streptomycin. SP2/0 cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 μg/mL of penicillin and 100 μg/mL of streptomycin. Hybridoma cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 μg/mL of penicillin, 100 μg/mL streptomycin, and HAT supplement (50×). The titers of EV71 strain C4 (China Center for Type Culture Collection (CCTCC) No. GDV103, PopSet No. KC954663.1) were determined using RD cells, which were expressed as the 50% tissue culture infectious dose (TCID50), according to the Karber method.
-
Antibodies were purified from cell culture supernatants using Protein A+G SepharoseTM (GE Healthcare), according to the manufacturer's instructions.
-
Concentrations of proteins or antibodies were measured by the Bradford method. Twenty nanograms of VP1 protein, inactivated EV71 virus, or PBS were coated on 96-well ELISA plates and incubated overnight at 4 ℃. Purifed mAbs (0.5 μg/well) were applied and incubated at 37 ℃ for 1 h, followed by administration of an anti-mouse IgG secondary antibody and the chemiluminescent substrate. Finally, the absorbance was measured at 450 nm.
-
Proteins samples (5 μg of VLPs, 10 μg of purified mAb, or 5 μg of inactivated EV71 virus) were loaded into a 10% SDS-polyacrylamide gel for electrophoresis. Samples were stained with Coomassie blue or transferred to PVDF membrane. For Western blot analysis, the membrane was successively immersed in 5% BSA at 4 ℃, incubated for 1 h with purifed mAbs (1.6 mg/mL) or a VP1-specifc antiserum, washed with TBST three times, incubated with anti-mouse IgG secondary antibodies conjugated with horseradish peroxidase, and washed in TBST a further three times. Finally, the proteins were visualized using SuperSignal West Pico Chemiluminescent Substrate.
-
The neutralization concentration of purifed mAb (1.6 mg/mL) was determined using a plaque reduction assay and a TCID50 reduction assay in RD cells. RD cells were cultured in 96-well plates overnight until 70% confluence. Antibodies were serially diluted two-fold with DMEM containing 2% FBS. One hundred microliters of EV71 virus containing 100 TCID50 was mixed with 100 μL of diluted mAb. After incubation at 37 ℃ for 1 h, the mixtures were added to the RD cells. After 6 days of culture, the lowest neutralization concentration was evaluated by observing the appearance of cytopathic effects (CPE).
Antigens, immunization of mice, and hybridoma generation
Cells, culture medium, and viruses
Antibody purifcation
Characterization of purifed antibodies by ELISA
SDS-PAGE and Western blot
Neutralization assay
-
The physical structure of the EV71-VLPs was determined using transmission electron microscope (TEM) analysis at the instrument center of the College of Life Sciences, Wuhan University. TEM images of negatively stained samples showed the presence of intact VLPs (Figure 1A), which had an icosahedral structure and which were morphologically similar to EV71 virus par-ticles (Wang et al., 2012). The SDS-PAGE assay showed the presence of three major structural proteins, VP0 (38 kDa), VP1 (36 kDa), and VP3 (27 kDa), of EV71 in the VLPs (Figure 1B). Thus, VLPs produced in yeast are of good quality and can be used as immunogens.
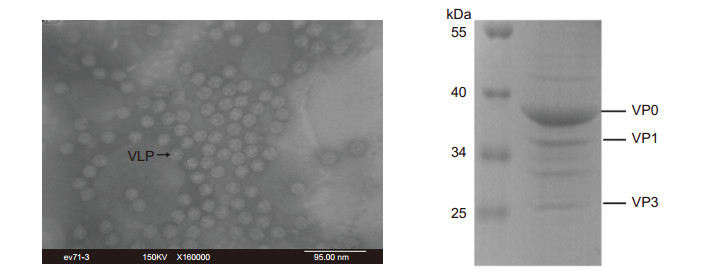
Figure 1. Virus-like particles were characterized using TEM examination (A) and SDS-PAGE (B). (A) Images of VLPs were analyzed by TEM at 160, 000× magnifcation. The VLPs were approximately 30 nm in diameter and were morphologically similar to empty particles of EV71. (B) SDS-PAGE assay demonstrated the presence of three EV71 capsid proteins (5 μg VLPs): VP0 (38 kDa), VP1 (36 kDa), and VP3 (27 kDa) in the VLPs.
-
The supernatant of the hybridoma cell culture was assayed by ELISA to evaluate its ability to bind to inactivated EV71 virus. The neutralization capacity of the positive supernatants was determined by an in vitro neutralization assay. Following four rounds of ELISA and neutralization assay screening, two hybridomas (D4 and G12) that could produce anti-EV71 antibodies that showed a strong reaction with the above antigens and that could effectively inhibit EV71 infection were identifed.
-
The purity and integrity of the antibodies that were purifed from the supernatant of the hybridoma D4 or G12 cell cultures with protein A+G SepharoseTM were analyzed by SDS-PAGE. The heavy chain (55 kDa) and light chain (25 kDa) molecules of both antibodies (mAb D4 and G12) were as expected (Figure 2A, lanes 1 and 2).
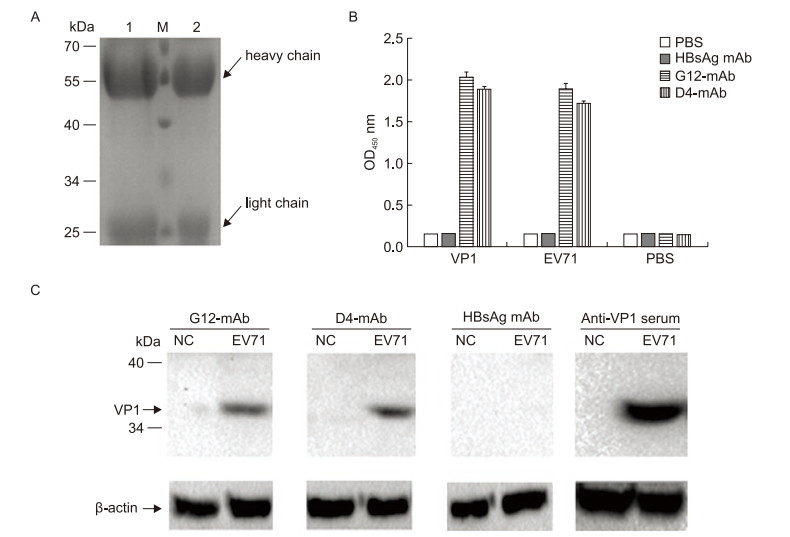
Figure 2. Relative titer of mAbs reacted with different antigens and specifcity testing of mAbs. (A) The purifed antiEV71 antibodies (10 μg per lane) were analyzed by SDS-PAGE. Lane M, protein size standards; Lane 1, mAb from G12 hybridoma cells (mAb G12); lane 2, antibodies from D4 hybridoma cells (mAb D4). (B) Triplicate samples (20 ng/well of VP1 protein, inactivated EV71 virus, or PBS) were added to a 96-well ELISA plate. Purifed antibody (0.5 μg/well) was applied and incubated at 37 ℃ for 1 h, followed by an anti-mouse IgG secondary antibody. Anti-HBsAg mAb and PBS were used as the negative control and the blank control, respectively. The average OD450 ± standard showed the relative titer. (C) Five micrograms of inactivated EV71 virus was resolved using a 10% SDS-PAGE gel, followed transfer to a PVDF membrane, probing with mAb D4, G12, HBsAg mAb, or VP1-specifc antiserum and incubated with anti-mouse IgG horseradish peroxidase-conjugated secondary antibodies. Uninfected RD cells were used as the negative control.
The D4 and G12 mAbs were then analyzed with ELISA to determine their relative titers with different antigens (VP1 protein, inactivated EV71 virus, or PBS). As shown in Figure 2B, both purifed mAbs reacted with the VP1 protein and the inactivated EV71 virus, but the anti-HBsAg negative control and the PBS blank control showed no signifcant reactivity with the antigens.
The specifcity of the D4 and G12 mAbs were evaluated by Western blot analysis. Comparison of VP1-specifc antiserum with control anti-HBsAg showed that both purifed mAbs could react with VP1 of EV71 positively and specifcally (Figure 2C).
-
The neutralizing effect of the purifed antibodies was tested using an in vitro neutralization assay. EV71 (100 TCID50) was incubated with serially diluted antibodies, followed by infection of RD cells. Uninfected RD cells were used as negative controls and infected RD cells with PBS were used as positive controls. As shown in Table 1, the lowest mAb concentration that could protect > 95% cells from CPE was 25 μg/mL. In contrast, RD cells infected with the virus alone showed severe CPE that resulted in cell rounding, aggregation, and flotation (Figure 3A).

Table 1. Neutralization capacity of purifed mAbs to inhibit CPE
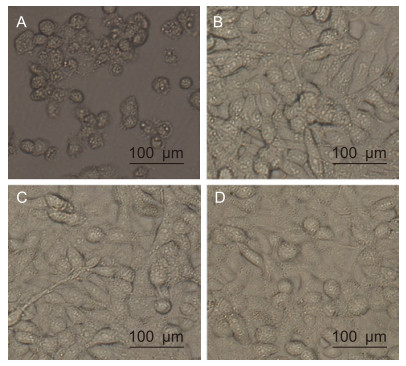
Figure 3. Effect of mAbs treatment on EV71-induced CPE. Cells were photographed at 6 days post-infection. (A) Infected RD cells incubated with PBS. (B) Uninfected RD cells. (C) pretreated EV71 with 25 μg/mL of mAb G12. (D) Pretreated EV71 with 25 μg/mL of mAb D4.
Uninfected RD cells grew normally (Figure 3B). As shown by the plaque reduction and TCID50 assays, pretreatment of the virus with the lowest concentration anti-EV71 antibodies (25 μg/mL of G12 or D4) protected > 95% of cells from CPE development (Figure 3C, mAb G12; Figure 3D, mAb D4). Thus, the two purifed antibodies have EV71-neutralizing effects.
Characterization of EV71-VLPs produced in yeast
Screening of hybridomas that secrete anti-EV71 antibodies
Purity, titer, and specifcity of anti-EV71 antibodies
Neutralizing effect of anti-EV71 D4 and G12 mAbs
-
Neutralizing antibodies against EV71 have been produced in mice immunized with live virus (Chang et al., 2011), inactivated virus (Chang et al., 2010), or recombinant VP1 protein (Wu et al., 2001; Chen et al., 2006; Sivasamugham et al., 2006; Chen et al., 2008; Li et al., 2009; Zhou et al., 2015). Live or inactivated virus can produce a high level of immunogenicity and protection. However, the safety of these treatments is controversial. Although the immunogenicity of recombinant VP1 protein is limited, due to a lack of suffcient epitopes, SP70 (VP1 208–222 aa) produces effective antibodies against EV71 but with a low neutralizing titer.
Virus-like particles (VLPs) are composed of viral capsid proteins, but lack the complete virus genome. VLPs have the same morphology and structure as viral capsid proteins. The immunogenicity of VLPs is usually similar to that of the live or inactivated virus but stronger than that of a single viral protein; their safety is as high as that of single protein antigens. Therefore, VLPs have become a crucial material for development of therapeutic approaches and vaccines against viruses (Roldao et al., 2010). Li et al. (2013) reported that EV71-VLPs, which were produced by expressing the P1 and 3CD proteins in a yeast cell expression system, could efficiently induce robust humoral and cellular responses in mice. IgG titer and cytokine (IFN-γ, IL-2, IL-4, and IL-6) levels were significantly increased. In addition, serum from immunized mice appeared to neutralize EV71.
In our study, we used highly purifed EV71-VLPs generated in yeast to stimulate mice for screening of neutralizing antibodies against EV71. Both ELISA and Western blot analyses showed that the neutralizing antibodies secreted from two strains of hybridoma cells (G12, D4) could effectively recognize EV71 capsid proteins and showed anti-EV71 capacity. Both antibodies could protect > 95% of cells from 100 TCID50 EV71 infection in 25 μg/mL solution. The results demonstrated that VLPs of EV71 can elicit the production of neutralizing antibodies in mice, and those two neutralizing mAbs may be promising candidates in development for mAbs to treat EV71 infection, and utilized as suitable reagents for use in diagnostic tests and biological studies.
-
This research has been supported by the grant from National Natural Science Foundation of China (No.31070147).
-
The authors declare that they have no conflict of interest. The whole study was approved by the ethics committee of the College of Life Sciences, Wuhan University, China (No.WDSKY0201303).
-
SY Lyu and T Lin designed the experiments. T Lin performed the experiments. SY Lyu, T Lin and LZ Xianyu analyzed the data. SY Lyu and T Lin wrote the paper. All authors have read and approved the fnal manuscript.













 DownLoad:
DownLoad: