-
Dear Editor,
Infectious bursal disease virus (IBDV) causes infectious bursal disease, a highly contagious immunosuppressive disease that affects young chickens and causes economic losses in the poultry industry worldwide. IBDV replicates mainly in actively dividing B lymphocytes within the bursa of Fabricius (BF), leading to immunosuppression in affected flocks (Mahgoub et al., 2012). Viral protein 2 (VP2), the only structural component of the IBDV icosahedral capsid, is the major antigen responsible for inducing protective immunity in the host (Mahgoub et al., 2012).
Canarypox virus (CNPV), which belongs to the Avipoxvirus genus, has been widely used to develop recombinant viral vectors for in vivo expression of antigens derived from mammalian pathogens (Weli and Tryland., 2011). CNPV-based vectors induce excellent protective immune responses with a favorable safety profile because they do not replicate in vaccinated animals. Consequently, several recombinant CNPVs are now commercial veterinary vaccines (Weli and Tryland., 2011). Despite the success of viral vectors for mammals, few studies have assessed the effectiveness of recombinant CNPV as a vaccine candidate for birds (Bublot et al., 2010; Zanetti et al., 2014). We previously constructed and characterized a recombinant CNPV expressing IBDV VP2 (CN048-VP2) and demonstrated its immunogenicity by intramuscular administration in specific pathogen free (SPF) chickens (Zanetti et al., 2014). Herein, we evaluated the replication status of CN048-VP2 and the protection induced against IBDV infection with single-dose subcutaneous administration in 1-day-old SPF chickens. The results demonstrated that CN048-VP2 could induce protection against IBDV infection in vaccinated SPF chickens.
To perform the experiments, embryonated SPF eggs from Instituto Rosenbusch (Argentina) were kept in an automatic incubator (Yonar, Argentina) until hatching. The chicks were housed in single cages with water and commercial chicken feed ad libitum.
To study the in vivo distribution of the CNPV viral vector, we conducted two trials. In the first trial, six 11-day-old SPF chickens were intramuscularly vaccinated in one leg with CN048-VP2 (106 plaque-forming units (PFU) mixed with 0.5% v/v Indian ink). Three birds were sacrificed at 1 and 3 days after vaccination, and muscle at the immunization site (identified by Indian ink), muscle from the non-injected leg, BF, and spleen were removed, weighed, and homogenized in phosphate buffer (50% w/v). Total DNA was extracted from organs using a QIAamp® DNA Mini Kit (QIAGEN, USA) and used as the template for PCR amplification of CNPV241 (primers P39F: 5′-AATAACACGACACAGCCGCAAG-3′, P39R: 5′-CAATTAATTAGATCGTGGTGGA-3′) or the glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene (primers GAPDH Fw: 5′-AGAACATCATCCCAGCGTCC-3′, GAPDH Rv: 5′-CGGCAGGTCAGGTCAACA-3′).
CNPV241 was detected in samples from the injected muscle at 1 day post-inoculation (pi) but not at 3 days pi or in samples obtained from other organs at any time (Figure 1A). In contrast, gapdh was amplified by PCR in all the analyzed samples, confirming sufficient DNA quantity and quality for detection (Figure 1B).
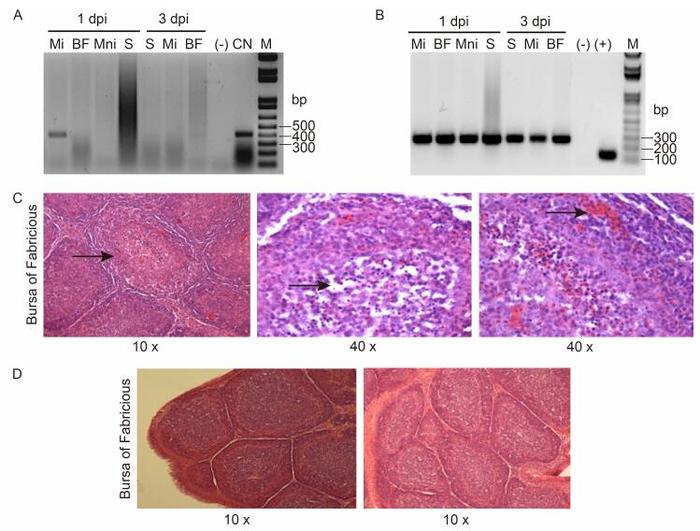
Figure 1. PCR amplification of viral CNPV241 (A) or chicken gapdh (B) genes using specific primers. Total DNA was purified from non-injected (Mni) or injected (Mi) muscle, bursa of Fabricius (BF), or spleen (S) of vaccinated chickens at 1 or 3 days post-injection. (-): without DNA, (+): plasmid carrying the intron-less gapdh gene (Carballeda et al, 2011), CN: purified CNPV DNA, M: molecular weight marker 1 kb plus.Photographs of tissue sections from hematoxylin-eosin stained bursae from chickens immunized with CNPV (C) or CN048-VP2 (D) and challenged with IBDV. Representative images (magnification 10-40×) from the BF are shown. The arrows indicate characteristic lesions induced by the challenge virus in the BF of chickens vaccinated with CNPV.
In the second experiment, two groups of two 25-day-old SPF chickens each were vaccinated with 500 PFU CN048-VP2 or fowlpox virus (FWPV) using a two-pronged wing-web applicator, and the emergence of nodular lesions or scabs at the site of inoculation was monitored daily. After FWPV vaccination, nodular lesions were clearly visible (3-5 mm diameter) at 5 days pi, and scabs appeared at 7 or 8 days pi. The lesions were re-adsorbed at 11 days pi. CN048-VP2 immunization produced 1-mm nodular lesions, without scabs, which were undetectable after 11 days (data not shown).
The efficacy of the vector was evaluated by subcutaneous vaccination of five 1-day-old SPF chicks with 106 PFU CNPV or CN048-VP2. Blood samples were obtained weekly after immunization to analyze the induced specific humoral response. After 7 weeks, chickens were orally challenged with 2×103 Tissue Culture Infectious Dose 50 (TCID50) of the IBDV LZD strain as previously described (Gómez et al., 2013). Five days later, the birds were sacrificed, and bursae were removed for analysis of protection parameters. VP2-specific antibody titers in individual serum samples were evaluated with the IDEXX FlockCheck® IBD ELISA kit (IDEXX Laboratories, USA), and pooled sera from each experimental group were analyzed using the in vitro IBDV neutralization assay described by Zanetti et al. (2012). Specific anti-CNPV antibody was detected by western blotting using CNPV sucrose cushion-purified virus as the antigen and an alkaline phosphatase-conjugated anti-chicken IgG (Sigma, USA) followed by BCIP/NBT substrate precipitation.
Anti-VP2 antibodies were undetectable in the sera of birds vaccinated with CN048-VP2 or CNPV throughout the experiment (data not shown). Similarly, the sera were negative for neutralizing activity to IBDV. In addition, no specific anti-CNPV antibodies were detected after single-dose immunization with CNPV-based vectors (data not shown).
Protection against IBDV infection was evaluated by analyzing mononuclear cell infiltration, the presence of IBDV, and histopathological lesions in the BF. Changes in immune cell frequency were analyzed by flow cytometry using mononuclear cells isolated from bursae and stained with different combinations of monoclonal antibodies, as described by Carballeda et al. (2011). The results are summarized in Table 1. Interestingly, bursae of birds vaccinated with CN048-VP2 revealed lower frequencies of infiltrated CD4+, CD8α+/β+, and KUL01+ cells (4.5-, 17-, and 1.6-fold, respectively) than samples from the control group (CNPV). In contrast, chickens immunized with CN048-VP2 displayed higher frequencies of B lymphocytes (Bu-1+) than those inoculated with CNPV.
CD4+ CD8α+/β+ KUL01+ Bu-1+ IBDV titers(PFU/mL) CN048-VP2 0.93 0.27 6.70 98.32 < 101 CNPV 4.36 4.57 10.52 88.34 6×106 Table 1. Analysis of cell subpopulations and IBDV titers in the bursa of Fabricius after challenge
The presence of IBDV in the bursa was analyzed by limiting dilution titration. Supernatants of bursa homogenates (50% w/v in sterile phosphate buffer) were pooled for each experimental group and serially ten-fold diluted. Monolayers of chicken embryo fibroblasts in 96-well plates were infected with the dilutions and incubated at 37℃ with 5% CO2 until a cytopathic effect was observed. Viral titers were calculated using the Reed and Muench method (1938). Whereas the IBDV LZD strain was present in the homogenates of chickens vaccinated with CNPV, the challenge virus was undetectable in bursae of chickens immunized with CN048-VP2 (Table 1).
Histopathological analysis of BF sections was performed with hematoxylin-eosin staining. Microscopic examination revealed histopathological lesions in the control group (CNPV-vaccinated chickens) such as loss of cortico-medullary definition, lymphoid depletion, and the presence of inflammatory infiltrate (Figure 1C). In contrast, tissue sections of birds immunized with CN048-VP2 showed no tissue damage (Figure 1D).
Vaccines used to control IBDV are generally based on attenuated strains of the pathogen. However, less-attenuated strains able to overcome passive immunity can damage the bursa. This immunosuppression interferes with the efficacy of other vaccines and promotes pathogen infections (Mahgoub et al., 2012). Turkey herpes virus (HVT) expressing IBDV-VP2 protein (Darteil et al., 1995) and an immune complex (IBDV strain with an anti-IBDV hyperimmune serum) (Müller et al., 2003) are also employed in commercial flocks. Although these vaccines are effective in the presence of IBDV maternal antibodies, they are expensive because they require a strict cold-chain to preserve the viability of HVT-infected cells or the addition of anti-serum (immune complex). Based on the need for safe and effective novel vaccines against IBDV, we evaluated the in vivo distribution and efficacy of the recombinant viral vector CN048-VP2. Our data revealed a safe profile of CN048-VP2 for vaccination of chickens, as the viral vector was unable to spread from the inoculation site. Moreover, the cutaneous lesions in wing-web induced by CN048-VP2 inoculation were minor relative to those generated by the replicative FWPV vaccine. To our knowledge, this is the first report confirming the non-replicative feature of CNPV in chickens. We also demonstrated that CN048-VP2 could induce protection against IBDV infection in vaccinated SPF chickens: T-cell infiltration was specifically decreased, and the percentage of premature B lymphocytes was higher than in chickens vaccinated with CNPV (control group), suggesting minor damage of this cell population in the target organ. These results correlate with the absence of both histopathological lesions and challenge virus in the BFs of the chickens immunized with CN048-VP2.
Since an IBDV-specific humoral response was not detected in the birds vaccinated with CN048-VP2, the protection observed could be mediated by the induction of cellular immunity. These results are consistent with previous reports that chickens vaccinated with FWPV expressing IBDV VP2 were protected against IBDV challenges without detectable anti-VP2 antibodies (Bayliss et al., 1991; Shaw et al., 2000). Thus, recombinant poxvirus can express heterologous antigens in the host cell cytoplasm that allow its processing and presentation with major histocompatibility complex molecules on the host cell surface for T lymphocyte recognition (Rocha et al., 2004).
In summary, our results demonstrated the non-replicative feature of the recombinant CNPV-based vector CN048-VP2 and its effective protection of SPF chickens against IBDV infection.
HTML
-
This work was supported by grants AERG 232141/PNBIO 1131032 from the Instituto Nacional de Tecnología Agropecuaria (INTA) and PICT 2008-0400 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Argentina. The authors acknowledge Dr. O. Zabal for preparing primary chicken embryo fibroblasts, Mrs. M.J. Monaco for technical assistance, and Mr. S. Díaz for animal care. We specially thank Dr. A. Venzano for histopathological examination of BFs, Dr. J.M. Carballeda and Dr. M.J. Gravisaco for assistance with flow cytometry analysis, and Dr. J. Sabio y García for English language editing. All institutional and national guidelines for the care and use of laboratory animals were followed. All protocols involving animals were approved and supervised by the Institutional Committee for the Care and Use of Experimental Animals (CICUAE-CICVyA-INTA). The authors declare that they have no conflict of interest.














 DownLoad:
DownLoad: