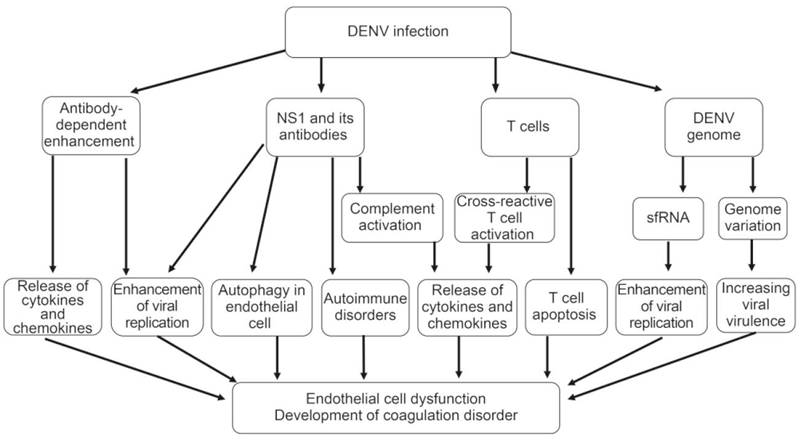
-
Alvarez M, Rodriguez-Roche R, Bernardo L, Vazquez S, Morier L, Gonzalez D, Castro O, Kouri G, Halstead SB, Guzman MG. 2006. Dengue hemorrhagic Fever caused by sequential dengue 1-3 virus infections over a long time interval: Havana epidemic, 2001-2002. Am J Trop Med Hyg, 75: 1113-1117.
-
Anderson JR, Rico-Hesse R. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg, 75: 886-892.
-
Avirutnan P, Zhang L, Punyadee N, Manuyakorn A, Puttikhunt C, Kasinrerk W, Malasit P, Atkinson JP, Diamond MS. 2007. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog, 3: e183.
doi: 10.1371/journal.ppat.0030183
-
Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. 2015. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med, 7: 304ra141.
doi: 10.1126/scitranslmed.aaa3787
-
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature, 496: 504-507.
doi: 10.1038/nature12060
-
Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. 2008. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis, 8: 86.
-
Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al-Afif A, Anderson R. 2011. Dengue virus infection of mast cells triggers endothelial cell activation. J Virol, 85: 1145-1150.
doi: 10.1128/JVI.01630-10
-
Chaichana P, Okabayashi T, Puiprom O, Sasayama M, Sasaki T, Yamashita A, Ramasoota P, Kurosu T, Ikuta K. 2014. Low levels of antibody-dependent enhancement in vitro using viruses and plasma from dengue patients. PLoS One, 9: e92173.
doi: 10.1371/journal.pone.0092173
-
Chan-Hui PY, Swiderek KM. 2016. Immunological considerations for developing antibody therapeutics for Influenza A. Hum Vaccin Immunother, 12: 474-477.
doi: 10.1080/21645515.2015.1079676
-
Chang RY, Hsu TW, Chen YL, Liu SF, Tsai YJ, Lin YT, Chen YS, Fan YH. 2013. Japanese encephalitis virus non-coding RNA inhibits activation of interferon by blocking nuclear translocation of interferon regulatory factor 3. Vet Microbiol, 166: 11-21.
doi: 10.1016/j.vetmic.2013.04.026
-
Chapman EG, Costantino DA, Rabe JL, Moon SL, Wilusz J, Nix JC, Kieft JS. 2014. The structural basis of pathogenic subgenomic flavivirus RNA (sfRNA) production. Science, 344: 307-310.
doi: 10.1126/science.1250897
-
Chen HL, Lin SR, Liu HF, King CC, Hsieh SC, Wang WK. 2008. Evolution of dengue virus type 2 during two consecutive outbreaks with an increase in severity in southern Taiwan in 2001-2002. Am J Trop Med Hyg, 79: 495-505.
-
Chen HR, Chuang YC, Lin YS, Liu HS, Liu CC, Perng GC, Yeh TM. 2016. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl Trop Dis, 10: e0004828.
doi: 10.1371/journal.pntd.0004828
-
Chuang YC, Lin J, Lin YS, Wang S, Yeh TM. 2016. Dengue Virus Nonstructural Protein 1-Ⅰnduced Antibodies Cross-React with Human Plasminogen and Enhance Its Activation. J Immunol, 196: 1218-1226.
doi: 10.4049/jimmunol.1500057
-
Chuang YC, Wang SY, Lin YS, Chen HR, Yeh TM. 2013. Re-evaluation of the pathogenic roles of nonstructural protein 1 and its antibodies during dengue virus infection. J Biomed Sci, 20: 42.
doi: 10.1186/1423-0127-20-42
-
Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science, 328: 745-748.
doi: 10.1126/science.1185181
-
Falconar AK. 1997. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol, 142: 897-916.
doi: 10.1007/s007050050127
-
Funk A, Truong K, Nagasaki T, Torres S, Floden N, Balmori Melian E, Edmonds J, Dong H, Shi PY, Khromykh AA. 2010. RNA structures required for production of subgenomic flavivirus RNA. J Virol, 84: 11407-11417.
doi: 10.1128/JVI.01159-10
-
Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. 2007. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A, 104: 9422-9427.
doi: 10.1073/pnas.0703498104
-
Green S, Pichyangkul S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Nisalak A, Kurane I, Rothman AL, Ennis FA. 1999. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis, 180: 1429-1435.
-
Gubler DJ. 2011. Emerging vector-borne flavivirus diseases: are vaccines the solution? Expert Rev Vaccines, 10: 563-565.
doi: 10.1586/erv.11.35
-
Gubler DJ, Suharyono W, Lubis I, Eram S, Gunarso S. 1981. Epidemic dengue 3 in central Java, associated with low viremia in man. Am J Trop Med Hyg, 30: 1094-1099.
-
Guzman MG, Alvarez M, Halstead SB. 2013. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol, 158: 1445-1459.
doi: 10.1007/s00705-013-1645-3
-
Guzman MG, Kouri G, Halstead SB. 2000. Do escape mutants explain rapid increases in dengue case-fatality rates within epidemics? Lancet, 355: 1902-1903.
doi: 10.1016/S0140-6736(00)02303-5
-
Halstead SB. 1970. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med, 42: 350-362.
-
Halstead SB. 1982. Immune enhancement of viral infection. Prog Allergy, 31: 301-364.
-
Halstead SB, Cohen SN. 2015. Dengue Hemorrhagic Fever at 60 Years: Early Evolution of Concepts of Causation and Treatment. Microbiol Mol Biol Rev, 79: 281-291.
doi: 10.1128/MMBR.00009-15
-
Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP. 2002. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis, 8: 1474-1479.
doi: 10.3201/eid0812.020170
-
Hermann LL, Gupta SB, Manoff SB, Kalayanarooj S, Gibbons RV, Coller BA. 2015. Advances in the understanding, management, and prevention of dengue. J Clin Virol, 64: 153-159.
doi: 10.1016/j.jcv.2014.08.031
-
Hladish TJ, Pearson CA, Chao DL, Rojas DP, Recchia GL, Gomez-Dantes H, Halloran ME, Pulliam JR, Longini IM. 2016. Projected Impact of Dengue Vaccination in Yucatan, Mexico. PLoS Negl Trop Dis, 10: e0004661.
doi: 10.1371/journal.pntd.0004661
-
Jacobs MG, Robinson PJ, Bletchly C, Mackenzie JM, Young PR. 2000. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J, 14: 1603-1610.
doi: 10.1096/fj.14.11.1603
-
Jaiyen Y, Masrinoul P, Kalayanarooj S, Pulmanausahakul R, Ubol S. 2009. Characteristics of dengue virus-infected peripheral blood mononuclear cell death that correlates with the severity of illness. Microbiol Immunol, 53: 442-450.
doi: 10.1111/mim.2009.53.issue-8
-
King CA, Anderson R, Marshall JS. 2002. Dengue virus selectively induces human mast cell chemokine production. J Virol, 76: 8408-8419.
doi: 10.1128/JVI.76.16.8408-8419.2002
-
Kurane I, Matsutani T, Suzuki R, Takasaki T, Kalayanarooj S, Green S, Rothman AL, Ennis FA. 2011. T-cell responses to dengue virus in humans. Trop Med Health, 39: 45-51.
doi: 10.2149/tmh.2011-S09
-
Kurosu T, Chaichana P, Yamate M, Anantapreecha S, Ikuta K. 2007. Secreted complement regulatory protein clusterin interacts with dengue virus nonstructural protein 1. Biochem Biophys Res Commun, 362: 1051-1056.
doi: 10.1016/j.bbrc.2007.08.137
-
Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis, 186: 1165-1168.
doi: 10.1086/jid.2002.186.issue-8
-
Lin CF, Chiu SC, Hsiao YL, Wan SW, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH, Liu CC, Lin YS. 2005. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J Immunol, 174: 395-403.
doi: 10.4049/jimmunol.174.1.395
-
Liu IJ, Chiu CY, Chen YC, Wu HC. 2011. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J Biol Chem, 286: 9726-9736.
doi: 10.1074/jbc.M110.170993
-
Luo YY, Feng JJ, Zhou JM, Yu ZZ, Fang DY, Yan HJ, Zeng GC, Jiang LF. 2013. Identification of a novel infection-enhancing epitope on dengue prM using a dengue cross-reacting monoclonal antibody. BMC Microbiol, 13: 194.
doi: 10.1186/1471-2180-13-194
-
Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS. 2012. Cellular and cytokine correlates of severe dengue infection. PLoS One, 7: e50387.
doi: 10.1371/journal.pone.0050387
-
Mammen MP, Lyons A, Innis BL, Sun W, McKinney D, Chung RC, Eckels KH, Putnak R, Kanesa-thasan N, Scherer JM, Statler J, Asher LV, Thomas SJ, Vaughn DW. 2014. Evaluation of dengue virus strains for human challenge studies. Vaccine, 32: 1488-1494.
doi: 10.1016/j.vaccine.2013.12.040
-
Manokaran G, Finol E, Wang C, Gunaratne J, Bahl J, Ong EZ, Tan HC, Sessions OM, Ward AM, Gubler DJ, Harris E, Garcia-Blanco MA, Ooi EE. 2015. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science, 350: 217-221.
doi: 10.1126/science.aab3369
-
Martina BE, Koraka P, Osterhaus AD. 2009. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev, 22: 564-581.
doi: 10.1128/CMR.00035-09
-
Martinez Gomez JM, Ong LC, Lam JH, Binte Aman SA, Libau EA, Lee PX, St John AL, Alonso S. 2016. Maternal Antibody-Mediated Disease Enhancement in Type Ⅰ Interferon-Deficient Mice Leads to Lethal Disease Associated with Liver Damage. PLoS Negl Trop Dis, 10: e0004536.
doi: 10.1371/journal.pntd.0004536
-
Mathew A, Rothman AL. 2008. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev, 225: 300-313.
doi: 10.1111/imr.2008.225.issue-1
-
Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwong P, Grimes JM, Yoksan S, Malasit P, Simmons CP, Mongkolsapaya J, Screaton GR. 2011. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol, 85: 410-421.
doi: 10.1128/JVI.01826-10
-
Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR. 2015. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med, 7: 304ra142.
doi: 10.1126/scitranslmed.aaa3863
-
Moon SL, Anderson JR, Kumagai Y, Wilusz CJ, Akira S, Khromykh AA, Wilusz J. 2012. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA, 18: 2029-2040.
doi: 10.1261/rna.034330.112
-
Nielsen DG. 2009. The relationship of interacting immunological components in dengue pathogenesis. Virol J, 6: 211.
doi: 10.1186/1743-422X-6-211
-
Pijlman GP, Funk A, Kondratieva N, Leung J, Torres S, van der Aa L, Liu WJ, Palmenberg AC, Shi PY, Hall RA, Khromykh AA. 2008. A highly structured, nuclease-resistant, noncoding RNA produced by flaviviruses is required for pathogenicity. Cell Host Microbe, 4: 579-591.
doi: 10.1016/j.chom.2008.10.007
-
Rachman A, Harahap AR, Widhyasih RM. 2013. The role of anti-dengue virus NS-1 and anti-protein disulfide isomerase antibodies on platelet aggregation in secondary dengue infection. Acta Med Indones, 45: 44-48.
-
Recker M, Vannice K, Hombach J, Jit M, Simmons CP. 2016. Assessing dengue vaccination impact: Model challenges and future directions. Vaccine, 34: 4461-4465.
doi: 10.1016/j.vaccine.2016.06.082
-
Rico-Hesse R, Harrison LM, Nisalak A, Vaughn DW, Kalayanarooj S, Green S, Rothman AL, Ennis FA. 1998. Molecular evolution of dengue type 2 virus in Thailand. Am J Trop Med Hyg, 58: 96-101.
-
Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Boshell J, de Mesa MT, Nogueira RM, da Rosa AT. 1997. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology, 230: 244-251.
doi: 10.1006/viro.1997.8504
-
Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, Gubler DJ, Bennett SN, van den Hurk AF. 2013. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One, 8: e68137.
doi: 10.1371/journal.pone.0068137
-
Roby JA, Pijlman GP, Wilusz J, Khromykh AA. 2014. Noncoding subgenomic flavivirus RNA: multiple functions in West Nile virus pathogenesis and modulation of host responses. Viruses, 6: 404-427.
doi: 10.3390/v6020404
-
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet, 380: 1559-1567.
doi: 10.1016/S0140-6736(12)61428-7
-
Sasaki T, Setthapramote C, Kurosu T, Nishimura M, Asai A, Omokoko MD, Pipattanaboon C, Pitaksajjakul P, Limkittikul K, Subchareon A, Chaichana P, Okabayashi T, Hirai I, Leaungwutiwong P, Misaki R, Fujiyama K, Ono K, Okuno Y, Ramasoota P, Ikuta K. 2013. Dengue virus neutralization and antibody-dependent enhancement activities of human monoclonal antibodies derived from dengue patients at acute phase of secondary infection. Antiviral Res, 98: 423-431.
doi: 10.1016/j.antiviral.2013.03.018
-
Sato R, Hamada N, Kashiwagi T, Imamura Y, Hara K, Nishimura M, Kamimura T, Takasaki T, Watanabe H, Koga T. 2015. Dengue Hemorrhagic Fever in a Japanese Traveler with Pre-existing Japanese Encephalitis Virus Antibody. Trop Med Health, 43: 85-88.
doi: 10.2149/tmh.2014-34
-
Schnettler E, Sterken MG, Leung JY, Metz SW, Geertsema C, Goldbach RW, Vlak JM, Kohl A, Khromykh AA, Pijlman GP. 2012. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and Mammalian cells. J Virol, 86: 13486-13500.
doi: 10.1128/JVI.01104-12
-
Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE, Jr. 2012. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol, 86: 2665-2675.
doi: 10.1128/JVI.06335-11
-
Srikiatkhachorn A. 2009. Plasma leakage in dengue haemorrhagic fever. Thromb Haemost, 102: 1042-1049.
-
Srikiatkhachorn A, Kelley JF. 2014. Endothelial cells in dengue hemorrhagic fever. Antiviral Res, 109: 160-170.
doi: 10.1016/j.antiviral.2014.07.005
-
Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. 2007. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost, 5: 2291-2299.
doi: 10.1111/jth.2007.5.issue-11
-
Suresh R, Chandrasekaran P, Sutterwala FS, Mosser DM. 2016. Complement-mediated
-
Takada A, Feldmann H, Ksiazek TG, Kawaoka Y. 2003. Antibody-dependent enhancement of Ebola virus infection. J Virol, 77: 7539-7544.
doi: 10.1128/JVI.77.13.7539-7544.2003
-
Ubol S, Chareonsirisuthigul T, Kasisith J, Klungthong C. 2008. Clinical isolates of dengue virus with distinctive susceptibility to nitric oxide radical induce differential gene responses in THP-1 cells. Virology, 376: 290-296.
doi: 10.1016/j.virol.2008.03.030
-
Ubol S, Halstead SB. 2010. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccine Immunol, 17: 1829-1835.
doi: 10.1128/CVI.00316-10
-
Vasilakis N, Shell EJ, Fokam EB, Mason PW, Hanley KA, Estes DM, Weaver SC. 2007. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology, 358: 402-412.
doi: 10.1016/j.virol.2006.08.049
-
Wan SW, Lu YT, Huang CH, Lin CF, Anderson R, Liu HS, Yeh TM, Yen YT, Wu-Hsieh BA, Lin YS. 2014. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS One, 9: e92495.
doi: 10.1371/journal.pone.0092495
-
WHO/TDR. 2009. Dengue guidelines for diagnosis, treatment, prevention and control. New Edition. WHO Press, Geneva.