HTML
-
The Bunyaviridae family of viruses includes five genera-Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus-with over 350 different viruses isolated to date. With the exception of hantaviruses, all bunyaviruses are transmitted by arthropods infecting diverse vertebrates, invertebrates, and plants (Tospovirus is the only genus that infects plants). Many bunyaviruses are important human pathogens, causing severe diseases including encephalitis, hepatitis, and hemorrhagic fever. Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne pathogen that was first described in China in 2009 (Yu et al., 2011) and later was also isolated in Japan (Takahashi et al., 2014) and Korea (Denic et al., 2011). A similar virus, named the Heartland virus, was reported in 2012 from two patients in Missouri, USA (McMullan et al., 2012). The clinical symptoms of SFTSV infections include fever, thrombocytopenia, gastrointestinal disorder, and leukocytopenia, with a fatality rate of 2%-30% based on different reports (Yu et al., 2011; Liu Q et al., 2014; Liu S et al., 2014). SFTSV is a novel phlebovirus that encapsidates three segments of negative-sense or ambisense RNA, named small (S), medium (M), and large (L) (Figure 1A). The S RNA encodes the nucleocapsid protein N and non-structural protein NS, the M RNA encodes the glycoproteins Gn/Gc, and the L RNA encodes the viral RNA-dependent RNA polymerase (Figure 1B). The glycoproteins Gn/Gc are incorporated into the envelope of the virus particles to orchestrate both the docking of the virus particles to host cells and the low pH-dependent fusion of the virus and cell membranes during endocytic entry (Figure 2). In this review, we will discuss recent progress towards understanding the entry mechanism of SFTSV.
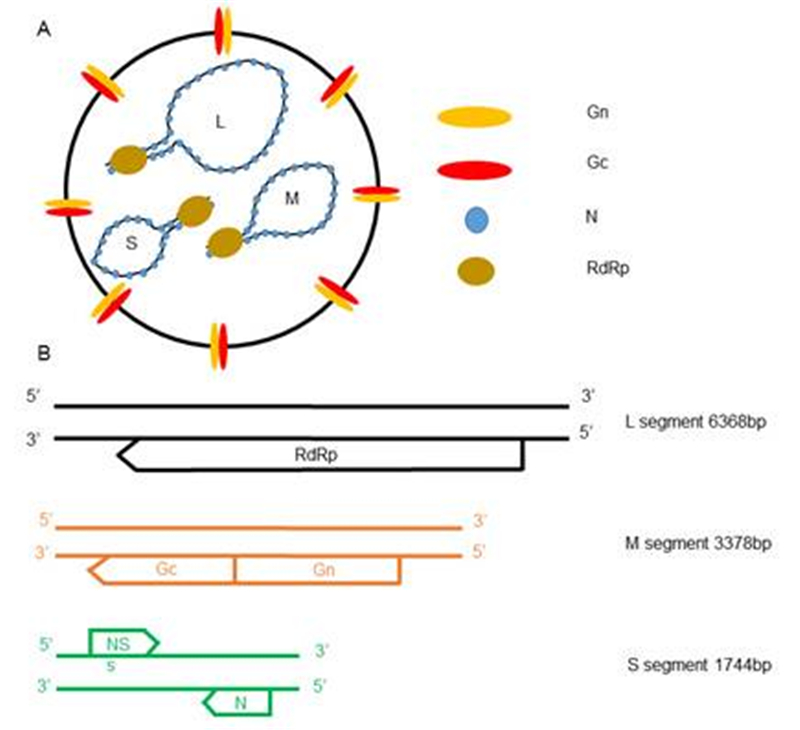
Figure 1. Schematic representation of the SFTSV virion and genome organization. (A) SFTSV particles are enveloped and spherical shaped, with the Gn-Gc dimer incorporated on the surface. The SFTSV genome consists of three segments, namely S/M/L, which are based on the size. (B) Arrows below each segment indicate the ORFs transcribed from the negative-sense templates, while the arrow above the segment shows the ORF transcribed from the positive-sense template.
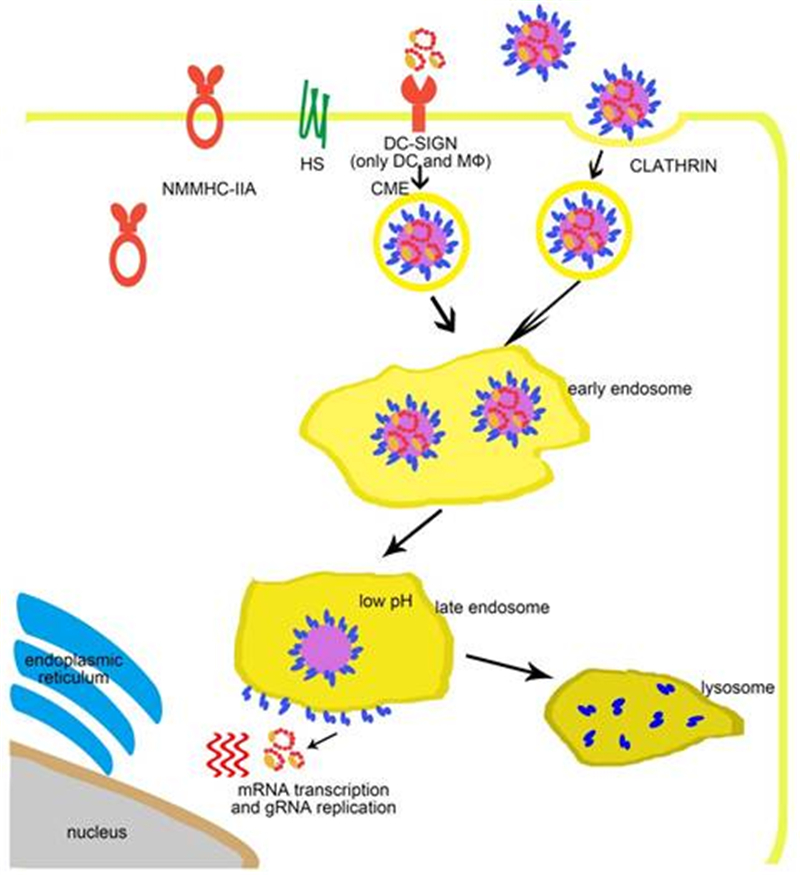
Figure 2. The entry process of SFTSV. SFTSV virus particles are attached to the cell membrane by interactions between glycoproteins and host cell factors including NMMHC-ⅡA, DC-SIGN, HS, and so far unknown factors. Virus particles are further internalized by the endocytosis pathway in a clathrin-dependent manner. In the late endosome, the low pH triggers the membrane fusion activity of the Gc glycoprotein, which allows the release of viral ribonucleoprotein complexes into the cytoplasm.
-
Bunyaviruses are enveloped, high-ordered spherical viruses with a diameter of 90-110 nm. The viral envelope is composed of a lipid bilayer and two transmembrane glycoproteins, Gn/Gc, regularly arranged on the surface. Although the SFTSV virion structure has barely been studied, electron cryotomography of the related phlebovirus Uukuniemi (UUKV) virus suggests that the spikes formed by Gn/Gc arrange in an icosahedral lattice, with T=12 triangulation (Overby et al., 2008). Two distinct spike organization forms have been discovered and are found to be pH-dependent, which is similar to the low pH-triggered conformational changes observed in alphaviruses and flaviviruses (Overby et al., 2008; Yu et al., 2008). The ribonucleoproteins (RNPs) formed by the RNA genome and nucleoprotein appear as a thread that partially interacts with the viral envelope (Overby et al., 2008). Similar results have also been reported for Rift Valley Fever Virus (RVFV), another phlebovirus. At a 2.1-2.3 nm resolution, the capsomers resemble hollow cylinders incorporated at five-and six-coordinated positions. The RNPs are also visible and strongly interact with the cytoplasmic tail of the glycoproteins, most likely Gn (Huiskonen et al., 2009).
-
The SFTSV glycoproteins mediate virus entry by binding to cellular receptors and inducing the fusion of the virus and the cell membrane during endocytosis. They are also the sole targets for neutralization antibodies. The M segment of the SFTSV encodes the Gn and Gc glycoproteins. In addition, the M segments of some bunyaviruses have also been found to encode an NSm protein; however, sequence analysis suggests that SFTSV does not have such a protein (Marklewitz et al., 2011; Yu et al., 2011). Like in other bunyaviruses, in SFTSV as well, Gn and Gc are first synthesized as a Gn/Gc precursor polyprotein in the secretory compartment of the infected cells, and the process then continues by proteolytic cleavage (Antic et al., 1992). The Gn/Gc precursor protein is cleaved by a signal peptidase during trafficking into the endoplasmic reticulum (ER) (Lober et al., 2001; Gerrard and Nichol, 2007) and then modified by N-linked glycosylation (Kuismanen, 1984). Upon trafficking to the Golgi apparatus, the glycan is further processed into hybrid and complex forms (Madoff and Lenard, 1982; Shi et al., 2005). When expressing Gn or Gc alone in the cell, Gn is transported to the Golgi apparatus owing to its Golgi localization motif, whereas Gc is localized to the ER owing to its ER retention signal (Gerrard and Nichol, 2002). However, when co-expressing Gn and Gc with a vaccinia virus vector, both Gn and Gc localize to the Golgi apparatus, which suggests that there a physical interaction occurs between Gn and Gc after cleavage (Wasmoen et al., 1988). Bunyavirus particles usually bud into the lumen of the Golgi apparatus during virus assembly, which is facilitated by Gn and Gc (Spiropoulou, 2001; Novoa et al., 2005; Overby et al., 2006; Piper et al., 2011; Cifuentes-Munoz et al., 2014).
-
The bunyavirus envelope glycoprotein Gc has been postulated to be a class Ⅱ fusion protein based on proteomic and computational analyses (Garry CE and Garry RF, 2004; Rusu et al., 2012). Consistent with this hypothesis, Gc of hantaviruses is found to interact with artificial membranes (Tischler et al., 2005). Dr. Yorgo Modis's laboratory has solved the crystal structure of the RVFV Gc protein, which shows a pre-fusion conformation of the class Ⅱ fusion protein previously found in alphaviruses and flaviviruses (Dessau and Modis, 2013). The extracellular domain of the RVFV Gc protein folds into a three-domain structure. Domain Ⅰ is a 10-stranded barrel located in the middle, the mostly stranded domain Ⅱ is extended from domain I, with the fusion loop on its tip, and domain Ⅲ is an IgC-like module connected to domain Ⅰ via a flexible linker (Dessau and Modis, 2013). The recently solved post-fusion structure of SFTSV Gc reveals the conformational changes triggered by low pH during virus entry (Halldorsson et al., 2016). Similar to the E1 protein of alphaviruses, the recombinant SFTSV Gc ectodomain is produced as a monomer at pH 8.0 (Pierson and Kielian, 2013). However, at pH 5.0, a trimer peak appears on size exclusion without interacting with artificial lipid membrane, as for most class Ⅱ fusion proteins (Dessau and Modis, 2013). The crystal structure produced from this peak at low pH reveals a clear class Ⅱ post-fusion conformation.
Comparison of the pre-fusion structure of RVFV Gc and post-fusion structure of SFTSV Gc reveals that there is a major conformational change occurring in the modules during fusion. Although the pre-and post-fusion conformations of DⅢ are very similar, major structural rearrangements are observed upon overlay of domains Ⅰ and Ⅱ (Dessau and Modis, 2013). Domain Ⅰ refolds into a secondary structure, which also occurs in the fusogenic rearrangement of the flavivirus (Modis et al., 2004) and alphavirus fusion proteins (Gibbons et al., 2004). Domain Ⅰ and Ⅱ of three monomers are packed into a trimer core, while domain Ⅲ shifts 24 Å toward the core, thus forming the outer layer of the Gc trimer (Dessau and Modis, 2013).
The mechanism underlying class Ⅱ membrane fusion has been intensively studied for alphaviruses and flaviviruses (Pierson and Kielian, 2013). The fusion process involves a series of conformational changes in the fusion protein triggered by the mildly acidic endosomal environment and includes several quick steps. Exposure of virus particles to low pH dissociates the E1/E2 (alphavirus) or the E2 (flavivirus) dimer and rotates the domain Ⅱ from the viral membrane. The fusion loop on the tip of domain Ⅱ is thus exposed and inserted into the host membrane. Domain Ⅰ/Ⅱ of E or E1 forms a trimer core via protein-protein interactions. DⅢ then rotates towards the trimer core and forms the outer layer of the E or E1 trimer. DⅢ also triggers the movement of the transmembrane domain, together with the viral membrane, towards the host membrane, thus resulting in the fusion of the virus and the host cell membranes (Kielian and Rey, 2006; Kielian et al., 2010).
-
Cellular receptor or receptors are the key host factors involved in the virus entry process. The interaction between viral glycoproteins and host receptors plays essential roles in determining cellular tropism, endocytosis, and/or activating the fusion process. To make the following discussion more clear, we defined virus receptors as the membrane components that precisely and specifically bind to viral glycoproteins and that are essential for viral entry. On the other hand, attachment factors were defined as membrane components that can enhance viral entry efficiency by interacting with viral particles but are ultimately dispensable for entry. Multiple factors such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), heparan sulfate (HS), and Nonmuscle Myosin Heavy Chain ⅡA (NMMHC-ⅡA) have been identified to be involved in the entry of SFTSV and other phleboviruses.
-
During natural transmission, bunyaviruses are injected into the skin during blood feeding by virus-carrying arthropods. At the initial infection, dermal dendritic cells (DCs) are among the first target cells. DC-SIGN is specifically expressed on the surface of dermal DCs and is a calcium-dependent C-type lectin specialized for the capture and presentation of foreign antigens (van Kooyk, 2008). DC-SIGN is a type Ⅱ transmembrane protein that binds with high-mannose N-glycans (Svajger et al., 2010), which are typical for insect-derived arbovirus glycoproteins. Therefore, alphaviruses and flaviviruses have been shown to infect immature DCs depending on DC-SIGN (Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003; Svajger et al., 2010). There are also many reports suggesting that DC-SIGN facilitates the entry of many phleboviruses including UUKV, RVFV, Punta Toro virus (PTV), Toscana virus (TOSV) (Lozach et al., 2011), La Crosse virus (LACV), and SFTSV (Hofmann et al., 2013). UUKV and RVFV infect DCs and DC-SIGN-expressing cell lines through the binding of DC-SIGN and high-mannose N-glycans on Gn/Gc proteins (Lozach et al., 2011). Upon virus inoculation, virus-induced clustering on the cell surface can be visualized by live cell imaging (Lozach et al., 2011). An endocytosis-defective mutant DC-SIGN does not mediate virus infection, which indicates that DC-SIGN is both an attachment factor and an authentic receptor. Upon docking on the cell membrane by binding to DC-SIGN, phleboviruses are delivered into cells due to the endocytotic signals in the cytoplasmic tail of DC-SIGN (Engering et al., 2002). In the early endosome, the virus particles dissociate from DC-SIGN and continue into the late endosome where membrane fusion happens (Lozach et al., 2011). A recent study has revealed that DC-SIGN, DC-SIGN-related (DC-SIGNR), and liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin) function as SFTSV receptors (Tani et al., 2016). However, there are many phlebovirus-susceptible cell lines that do not express lectins (Lozach et al., 2011), which indicates that other unidentified receptors for phlebovirus entry must exist. The presence of such receptors is also strongly suggested by the broad cell tropism of phleboviruses and the very limited tissue expression of DC-SIGN and other lectins.
-
All eukaryotic cells are covered by a dense and diverse layer of carbohydrates, which is essential for many biological processes (Varki, 2007). It is not a surprise that many viruses have evolved to exploit these ubiquitous and accessible surface glycans to promote their entry process (Liu and Thorp, 2002). Among these different types of glycans, HS serves as an attachment factor for many viruses (Liu and Thorp, 2002) including RVFV (de Boer et al., 2012). HS is a glycosaminoglycan (GAG), i.e., a linear polysaccharide that can be attached to membrane proteins to form proteoglycans. HS is abundantly expressed on most cell lines (Liu and Thorp, 2002). Using CHO cell lines with defined glycosylation defects, HS was revealed to be required for the efficient entry of RVFV. RVFV entry was also found to be significantly reduced by pre-incubating the virus with sulfated heparin or pre-treating the cells with heparinase (de Boer et al., 2012). Although RVFV infection on HS-defective cell lines was dramatically reduced, residual infection was still observed, which suggested that HS is more likely an attachment factor rather than a receptor (de Boer et al., 2012). The involvement of HS in RVFV entry has also been confirmed by a haploid screening conducted for RVFV spreading factor (Heijink et al., 2015). More evidence suggests that CCHFV and Hantaan virus (HNTV) also rely on HS for efficient entry, while Andes virus (ANDV) does not (Heijink et al., 2015). However, the involvement of HS in SFTSV entry is still under investigation.
-
NMMHC-ⅡA is an actin-binding motor protein that is normally involved in cell migration, adhesion, polarization, and morphogenesis (Vicente-Manzanares et al., 2009). NMMHC-ⅡA has also been shown to function as a herpes simplex virus type 1 (HSV-1) receptor, and NMMHC-ⅡA expression levels on the cell surface are increased upon HSV-1 infection (Arii et al., 2010). In 2014, Sun et al. reported that the extracellular domain of recombinant Gn protein binds to SFTSV-susceptible cell lines and blocks infection. Using Gn as bait, the authors identified NMMHC-ⅡA as an SFTSV entry factor by using a co-ⅠP strategy (Sun et al., 2014). The authors also found that overexpression of NMMHC-ⅡA enhanced SFTSV infection in HeLa cells. Moreover, RNAi knockdown of NMMHC-ⅡA and the use of an anti-NMMHC-ⅡA antibody were found to reduce SFTSV infection but did not completely block it (Sun et al., 2014). These data suggested that NMMHC-ⅡA is an attachment factor. As NMMHC-ⅡA is highly expressed on platelets and is essential for their normal functions, it is plausible that NMMHC-ⅡA directly contributes to the pathogenesis of SFTSV (Sun et al., 2014). An in vitro study of primary cells suggested that SFTSV virus particles can efficiently infect macrophages but can only bind to platelets. Another study revealed that SFTSV adheres to platelets and promotes the phagocytosis of SFTSV-platelet complexes by macrophages, thus resulting in thrombocytopenia (Jin et al., 2012). Collectively, these results indicate that NMMHC-ⅡA might play important roles in SFTSV entry and pathogenesis; however, the bona fide receptor for SFTSV entry is still unknown.
(1) DC-SIGN
(2) HS
(3) NMMHC-ⅡA
-
The fusion of SFTSV with the host cell membrane requires pH lower than 6 (Hofmann et al., 2013; Tani et al., 2016) A prerequisite for the activation of the SFTSV fusion protein Gc is the uptake of virions into the host cell endosomes (Tani et al., 2016). In a previous study, inhibitor analysis revealed that SFTSV entry could be blocked by dynasore, a dynamin inhibitor, which indicated that SFTSV entry depends on clathrin (Hofmann et al., 2013). Orthobunyavirus and Oropouche viruses also enter host cells via clathrin-coated pits (Santos et al., 2008; Hollidge et al., 2012). However, a different detailed study of the entry of UUKV suggested that UUKV internalization is mainly clathrin independent (Lozach et al., 2010). The authors reported that the virus enters Rab5a+ and LAMP-1+ late endosomes, where acid-activated penetration happens (Lozach et al., 2010). Together, these data suggest that different bunyaviruses use different internalization mechanisms for their entry, and the detailed processes involved in the same remain to be investigated.
-
Substantial progress has been made in understanding the entry mechanism of SFTSV after its discovery as an emerging virus in 2009. However, the discovery of the SFTSV Gc protein as a class Ⅱ fusion protein will especially provide important insights into the membrane fusion mechanism of the whole Bunyaviridae family. However, the critical receptor that determines cell tropism and entry is still unknown, although many attachment factors have been identified. Therefore, more efforts should be devoted to SFTSV research in the future.
-
This work was funded by the National Key Plan for Scientific Research and Development of China (2016YFD0500300), the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDB11030800), and the Natural Science Foundation of China (L1524009).
-
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: