-
Japanese encephalitis virus (JEV) is a member of the Flavivirus genus and spreads via mosquitoes (van den Hurk et al., 2009). As the major cause of viral encephalitis among children in Asia, JEV infection can lead to permanent neurological sequelae or even death due to the lack of a specific treatment (Iwasaki et al., 1986). JEV can replicate in different types of primary cell and in immortalized cell lines from a variety of species (Pierson et al., 2013). The permissiveness of T lymphocytes to JEV is well documented. For instance, a previous study showed that a small fraction of T lymphocytes and macrophages in mice was targeted during acute JEV infection (Aleyas et al., 2009). JEV can establish a latent infection in T lymphocytes of the spleen in mice that can be reactivated during pregnancy or by cyclophosphamide treatment (Mathur et al., 1989; Mathur et al., 1986). Clinical studies have shown that three out of 40 children with acute JE developed recurrence of the disease 8–9 months later due to virus recovered from T lymphocytes; in fact, JEV was also isolated from the T lymphocytes of asymptomatic children (Sharma et al., 1991). The virus likely requires early-stage dissemination from sites of infection in the skin to secondary lymphoid organs. However, it is unclear how JEV infects T lymphocytes.
Dendritic cells (DCs) are professional antigen-presenting cells that act as a bridge between peripheral mucosal and lymphoid tissues (Malissen et al., 2014). It has been reported that flaviviruses-infected DCs migrate to draining lymph nodes where efficient viral replication takes place, leading to viral entry into the circulation and internal organs (Diamond et al., 2003; Pierson et al., 2013). We speculate that the antigen-presenting and migratory capabilities of DCs are exploited by JEV for transfer of the virus to T cells in a mechanism similar to that used by human immunodeficiency virus (HIV)-1, in which DCs capture viruses and promote trans-infection of target cells expressing CD4 and a co-receptor (Geijtenbeek et al., 2000a).
Immature DCs cultured from monocytes in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 express high levels of DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), which meditates DC interactions with T lymphocytes through recognition of intercellular cell adhesion molecule (ICAM)-3 (Geijtenbeek et al., 2000a; Shimauchi et al., 2015). DC-SIGN is thought to play an important role in mediating trans-infection of T cells by several viruses, including HIV-1, cytomegalovirus, enterovirus 71, and measles virus (Geijtenbeek et al., 2000b; Halary et al., 2002; de Witte et al., 2008; Ren et al., 2014). We previously reported that DC-SIGN can efficiently capture and concentrate viral particles at the cell surface and thus facilitate cis-infection of JEV (Wang et al., 2016), but its role in promoting trans-infection of T cells is not known. This was investigated in the present study using DCs-T cells co-culture system and live-cell spinning-disk confocal microscopy. Our results demonstrate that DC-SIGN plays an important role in mediating the transmission of JEV from DCs to T cells.
-
C6/36 cells (provided by Professor Hanzhong Wang, Wuhan Institute of Virology, CAS) were grown in Eagle’s minimal essential medium (ATCC, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) at 28 °C and 5% CO2. Baby hamster kidney cells (BHK-21) were grown in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen), 100 U/mL penicillin-streptomycin, and 2 mmol/L L-glutamine at 37 °C and 5% CO2.
Primary DCs and T cells were obtained as previously described (Jin et al., 2014; Li et al., 2014). Briefly, human peripheral blood mononuclear cells (PBMCs) were isolated from blood samples collected from healthy adult volunteers by the standard Ficoll-Paque method. To obtain immature DCs, monocytes were isolated from PBMCs by negative selection using the Myeloid Dendritic Cell Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified monocytes were then differentiated into immature DCs in the presence of IL-4 (500 U/mL) and GM-CSF (800 U/mL). The cells were harvested on day 6 and analyzed by fluorescence-activated cell sorting (FACS) on a FACSAriaIII flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and used in subsequent assays. To prepare T cells, isolated PBMCs were activated with phytohemagglutinin (PHA, 1 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) and cultured in complete Roswell Park Memorial Institute (RPMI)-1640 medium. On day 3, the cells were washed and cultured with IL-2 (100 U/mL). PHA-activated PBMCs were harvested on day 6 as T cells. JEV strain SA14-14 was propagated in C6/36 cells and titered in BHK-21 cells. Viruses were concentrated and purified by ultracentrifugation. Briefly, 25 mL of virus-containing medium (cleared by centrifugation and filtration) was overlaid on 5 mL of 25% sucrose in conical tubes and centrifuged at 25,000 rpm for 2 h at 4 °C in an SW28 swinging bucket rotor (Beckman Coulter, Brea, CA, USA). The resultant pellet was dissolved in 100 μL/tube phosphate-buffered saline (PBS) without calcium or magnesium (Invitrogen) for 2 h at 4 °C. Aliquots were stored at –80 °C or were immediately purified by overlaying the concentrated virus on a continuous sucrose gradient (20%–55% w/w in PBS) and centrifuging at 35,000 rpm for 2 h at 4 °C in an SW55 swinging bucket rotor (Beckman Coulter).
JEV was labeled with Alexa Fluor® 488 Carboxylic Acid (Invitrogen) in HEPES (20 mmol/L) according to the manufacturer’s instructions. Labeled JEV was designated as JEV-AF488. Free dye was removed by passage through a gel filtration column and the virus was banded on a sucrose gradient as described above. Labeling was verified by SDS-PAGE, and infectivity was monitored with the plaque formation assay. Labeled JEV was exposed to ultraviolet light before use for live-cell confocal microscopy.
-
Mouse anti-DC-SIGN neutralizing monoclonal antibody (mAb; mAb161) and corresponding isotype control antibodies were purchased from R&D Systems (Minneapolis, MN, USA). Mouse anti-β-actin and horseradish peroxidase (HRP)-conjugated goat-anti-mouse antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse polyclonal antibody against domain III of JEV E glycoprotein was generated by immunizing BALB/c mice (BHK Ltd., Beijing, China) with JEV E DIII protein (a gift from Professor Gengfu Xiao, Wuhan Institute of Virology, CAS). Briefly, mice were subcutaneously injected with 200 μg of JEV E DIII protein in Freund’s complete adjuvant, followed by two boost immunizations with the same amount of antigen in Freund’s incomplete adjuvant at 3-week intervals. Two weeks after the final immunization, blood samples were collected and sera were pooled and purified with protein A/G (Invitrogen). EDTA and mannan were purchased from Sigma-Aldrich. Alexa Fluor® 488 Carboxylic Acid was purchased from Invitrogen.
-
Protein extracts were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane that was probed with antibodies against JEV E (1:2000) and β-actin (1:1000, Santa Cruz Biotechnology). Immunoreactivity was detected with HRP-conjugated goat anti-mouse IgG antibody (1:5000; Santa Cruz Biotechnology) and enhanced chemiluminescence western blotting substrate (Thermo Fisher Scientific, Waltham, MA, USA).
-
Cells were incubated with JEV or JEV-AF488 for 1 h on ice at a multiplicity of infection (MOI) of 10 in binding buffer composed of RPMI-1640 (pH 7.4), 0.2% FBS, 1 mmol/L CaCl2, and 2 mmol/L MgCl2. After washing with cold PBS containing 1% bovine serum albumin to remove unbound viruses, binding was quantified by FACS. DCs were seeded in poly-L-lysine-coated wells and incubated for 1 h at 37 °C followed by incubation with JEV-AF488. For the inhibition assay, cells were pretreated with inhibitors for 30 min before exposure to viruses.
-
DCs (1 × 106) were pulsed with JEV-AF488 (3 × 107 PFU) in binding buffer for 1 h on ice, then washed three times to remove unbound viruses and incubated with T cells (3 × 106) for predetermined times. T cells were labeled with CellTracker Blue fluorescence dye (C2110; Invitrogen) prior to incubation with DCs. Viral transmission was quantified by FACS or analyzed by fluorescence microscopy. T cells (3 × 106) pulsed with JEV-AF488 (3 × 107 PFU) served as the control. For the transmission inhibition assay, DCs were pretreated with inhibitor for 30 min before exposure to viruses and then incubated with T cells. In the non-contact co-culture system, T cells (3 × 104) were seeded in the upper chambers of an 8-μm 24-well Transwell plate (Corning, NY, USA); the lower chamber was seeded with JEV-pulsed DCs (1 × 104). For the transmission inhibition assay, DCs were pretreated with inhibitors for 30 min before exposure to viruses and then co-cultured with T cells. Transmission efficiency was determined by titration of the supernatant and by western blot analysis of viral protein E. For analysis of T cell trans-infection, T cells were labeled with CellTracker Blue fluorescent dye (C2110; Invitrogen) before mixing with JEV-AF488-pulsed DCs. Cells were co-cultured at 37 °C for 3 h, fixed in 1% formaldehyde, and then evaluated by FACS. Data were analyzed using BD FACSDiva software (BD Biosciences).
-
DCs were seeded in 3.5-cm diameter glass-bottomed culture dishes (MatTek, Ashland, MA, USA) pre-coated with poly-L-lysine. After pulsing with JEV-AF488 on ice, DCs were mixed with T cells at a ratio of 1:3. Dishes were then transferred to a chamber in which temperature, humidity, and CO2 levels were controlled during imaging. Time-lapse live cell imaging was performed on an Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) using a 60 × NA 1.4 oil objective or on a spinning-disk confocal microscope (UltraView VoX; PerkinElmer, Waltham, MA, USA). Images were acquired with excitation at 488 nm in differential interference contrast mode. Image squences were processed and analyzed using Volocity software (PerkinElmer, Waltham, MA, USA).
-
Data were analyzed with Prism v.5.00 software (GraphPad Inc., La Jolla, CA, USA). Values are reported as the mean ± SD of triplicate samples unless otherwise specified. Differences between groups were evaluated with the Student’s t-test. A P value < 0.05 was considered statistically significant.
-
To determine whether JEV exploits DCs to gain access to lymph nodes for efficient replication and subsequent transmission, we examined the transfer of DC-captured JEV to T cells. Monocytes were isolated from PBMCs and differentiated into DCs in the presence of IL-4 (500 U/mL) and GM-CSF (800 U/mL) (Supplementary Material S1A). Surface expression of DC-SIGN was detected by flow cytometry (Supplementary Material S1B). AF488 fluorescent dye labeling was used to track JEV particle movement in DC-T cell co-cultures. Infection of JEV-AF488 in DC-SIGN-expressing cells was similar to that of unlabeled JEV(Supplementary Material S2), indicating that the fluorescent dye labeling did not compromise virus infectivity. T cells were labeled with CellTracker Blue fluorescent dye before pulsing with JEV-AF488 (cell-free virus) at an MOI of 10 or with DC-associated JEV-AF488 for 30 min; JEV transmission was then quantified by flow cytometry by analyzing the CellTracker Blue+ JEV-AF488+ population. The binding efficacy of cell-free virus to CellTracker Blue-labeled T cells (i.e., cis-infection, in cis) was low even at an MOI of 10. When DCs were incubated with the same amount of virus followed by co-culturing with T cells (i.e., trans-infection, in trans), the binding efficacy of cell-associated virus was higher than that of cell-free virus (Figure 1A, 1B). These results indicate that JEV can be transmitted from DCs to T cells in trans.
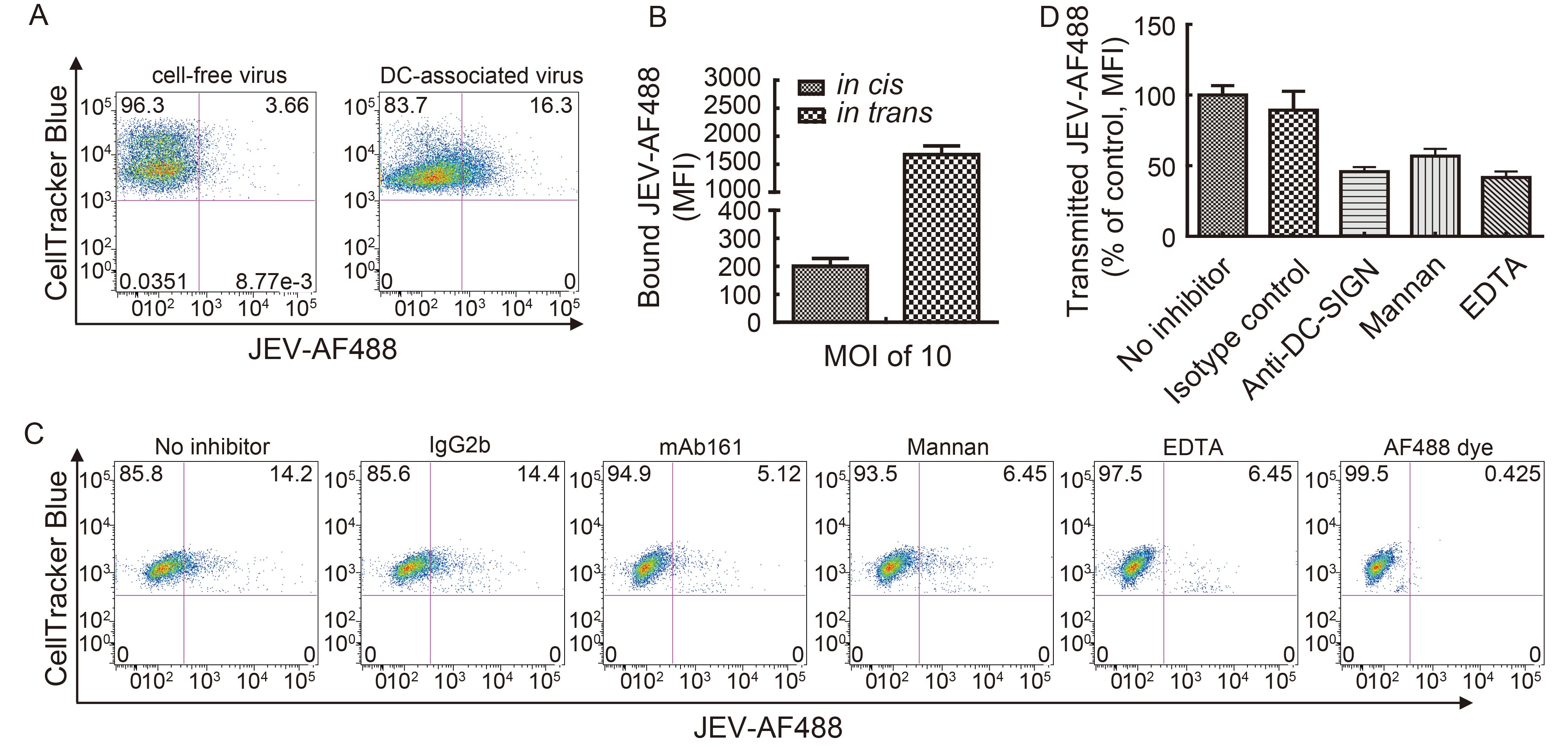
Figure 1. DC-captured JEV is transferred to T cells. (A) Transmission of cell-free (in cis) and DC-associated (in trans) JEV-AF488 to T cells. DCs were pulsed with JEV-AF488 (MOI = 10) on ice for 1 h and washed extensively to remove unbound viruses. T cells were labeled with CellTracker Blue fluorescent dye before pulsing with JEV-AF488 (cell-free virus, MOI = 10) or DC-associated JEV-AF488 for 30 min. JEV transmission was quantified by analyzing the CellTracker Blue+ JEV-AF488+ population. Results of a representative experiment (n = 3) are shown. (B) Transmission of JEV-AF488 in cis and in trans was assessed by measuring mean fluorescence intensity (MFI). Data represent mean ± SD of three independent experiments. (C) Transmission of JEV-AF488 in trans in the presence of C-type lectin inhibitors. DCs pulsed with JEV-AF488 were incubated with CellTracker Blue-labeled T cells in the presence of inhibitors for 30 min, fixed, and analyzed by FACS. T cells were first gated according to forward and side scatter characteristics, followed by analysis of CellTracker Blue+ JEV-AF488+ cells. Results of a representative experiment (n = 3) are shown. (D) Transmission of JEV-AF488 to T cells in trans in the presence of inhibitors was assessed by measuring MFI. Data represent mean ± SD of three independent experiments.
The C-type lectin receptor DC-SIGN is expressed at high levels on the surface of immature DCs and can mediate their interactions with T lymphocytes via recognition of ICAM-3 (Geijtenbeek et al., 2000a; Figdor et al., 2002). DC-SIGN plays an important role in mediating trans-infection of T cells by several viruses (Geijtenbeek et al., 2000a, 2000b; Halary et al., 2002; de Witte et al., 2008; Ren et al., 2014). Based on our demonstration that DC-SIGN is an attachment receptor for JEV and enhances DC infection in cis (Wang et al., 2016), we speculated that it participates in DC-mediated transfer of JEV to T cells. To evaluate this possibility, monocyte-derived DCs were pre-treated with specific blocking antibodies against DC-SIGN (mAb161) or with C-type lectin inhibitors (mannan and EDTA) as described in our previous studies (Wang et al., 2016); CellTracker Blue+ JEV-AF488+ populations were then analyzed by flow cytometry. The fraction of CellTracker Blue+ JEV-AF488+ cells was about 14% after co-culturing with JEV-pulsed DCs for 3 h, and virions transmitted to T cells were unaffected by pretreatment with isotype control antibodies but were inhibited by anti-DC-SIGN mAb161, EDTA, or mannan treatment (Figure 1C, 1D), indicating that DC-SIGN plays an important role in mediating the transmission of JEV from DCs to T cells in trans.
-
Given that DC-SIGN increased the transfer of JEV particles from DCs to T cells, we investigated whether co-culturing DCs and T cells would increase JEV infection. To mimic in vivo conditions in which JEV levels are likely to be low, DCs were treated with low titers of JEV (MOI = 0.001) and then co-cultured with T cells for 72 h (Figure 2A-a). DCs or T cells treated with equivalent amounts of JEV but without co-cultivation served as controls. As expected, neither DCs nor T cells alone were efficiently infected by the low titers of JEV. In contrast, more viral proteins (Figure 2B) and progeny virions (Figure 2C) were produced in T cells co-cultured with JEV-pulsed DCs. This effect was blocked by treatment with DC-SIGN inhibitors (Figure 2B, 2C), indicating that enhanced JEV infection in DC-T cell co-cultures is DC-SIGN-dependent—that is, DC-SIGN endows DCs with the ability to capture and transfer JEV particles to T cells, which promotes JEV replication in the DC-T cell microenvironment.
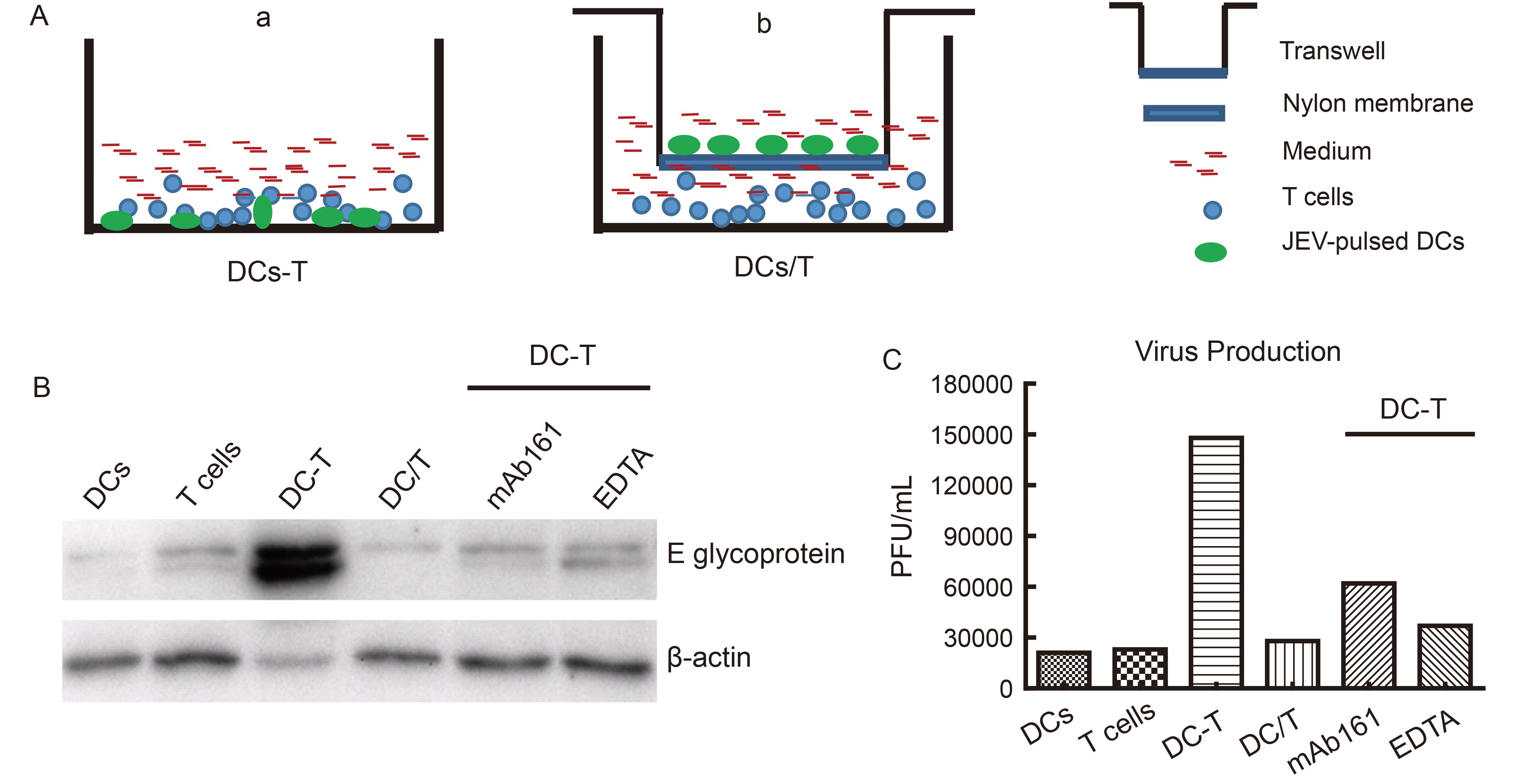
Figure 2. DC-SIGN is required for efficient JEV infection in DC-T co-cultures. DCs were infected with JEV at a low titer (MOI = 0.001) in the absence or presence of C-type lectin inhibitors and then co-cultured in contact (DC-T) or without contact (DC/T) with T cells for 72 h. JEV-infected DCs or T cells without co-culturing served as controls. (A-a) Model of DC-T. (A-b) Model of DC/T. (B) Intracellular viral proteins were detected by western blotting. (C) The progeny virus particles of the parallel samples of (B) were collected and evaluated. Results of a representative experiment (n = 3) are shown.
The contact area between a T cell and a DC is known as the immunological synapse (Norcross 1984; Paul et al., 1987; Shimauchi et al., 2015). Several viruses use a similar structure known as a virological synapse (VS) as a bridge for transmission from DCs to T cells. For instance, DC-SIGN-mediated VS formation enhances HIV-1 transmission from DCs to T cells (Felts et al., 2010). To determine whether cell-to-cell contact between DCs and T cells is necessary for JEV transfer, JEV-pulsed DCs were co-cultured with T cells in a non-contact system in which a semi-permeable transwell membrane prevented direct contact between the two types of cell but allowed free passage of cell-free viruses (i.e., DC/T co-cultures; Figure 2A-b). We compared the infection of JEV in the DC-T co-cultures with those in DCs or T cells cultured alone. Compared to that in the DC-T co-cultures, JEV replication was reduced in DC/T co-cultures (Figure 2B, 2C), implying a requirement of direct cell-to-cell contact for the transfer of DC-captured JEV particles to T cells.
-
To assess whether JEV particles were transferred from DCs to T cells via VS formed at the junction between the two cell types, DCs were exposed to JEV-AF488, co-cultured with CellTracker Blue-labeled T cells, and then immediately subjected to confocal microscopy analysis. Live-cell images revealed that T cells moved randomly and “scanned” the surface of DCs, followed by the formation of junctions (Figure 3A) and viral particle transmission, as reported by others (Arrighi et al., 2004; Dale et al., 2011). VS formation was quantified using flow cytometric analysis by determining the percentage of JEV-AF488+ CellTracker Blue+ DC-SIGN+ cell clusters. JEV binding-induced VS formation was rapid and was maintained over time. VS appeared as early as 5 min post co-cultivation, and most of them formed between 5 and 15 min (Supplementary Material S3). Viral particles typically moved to the VS between DCs and T cells and were then directionally transferred to T cells (Figure 3B, see video in Supplementary Material S4–S5).
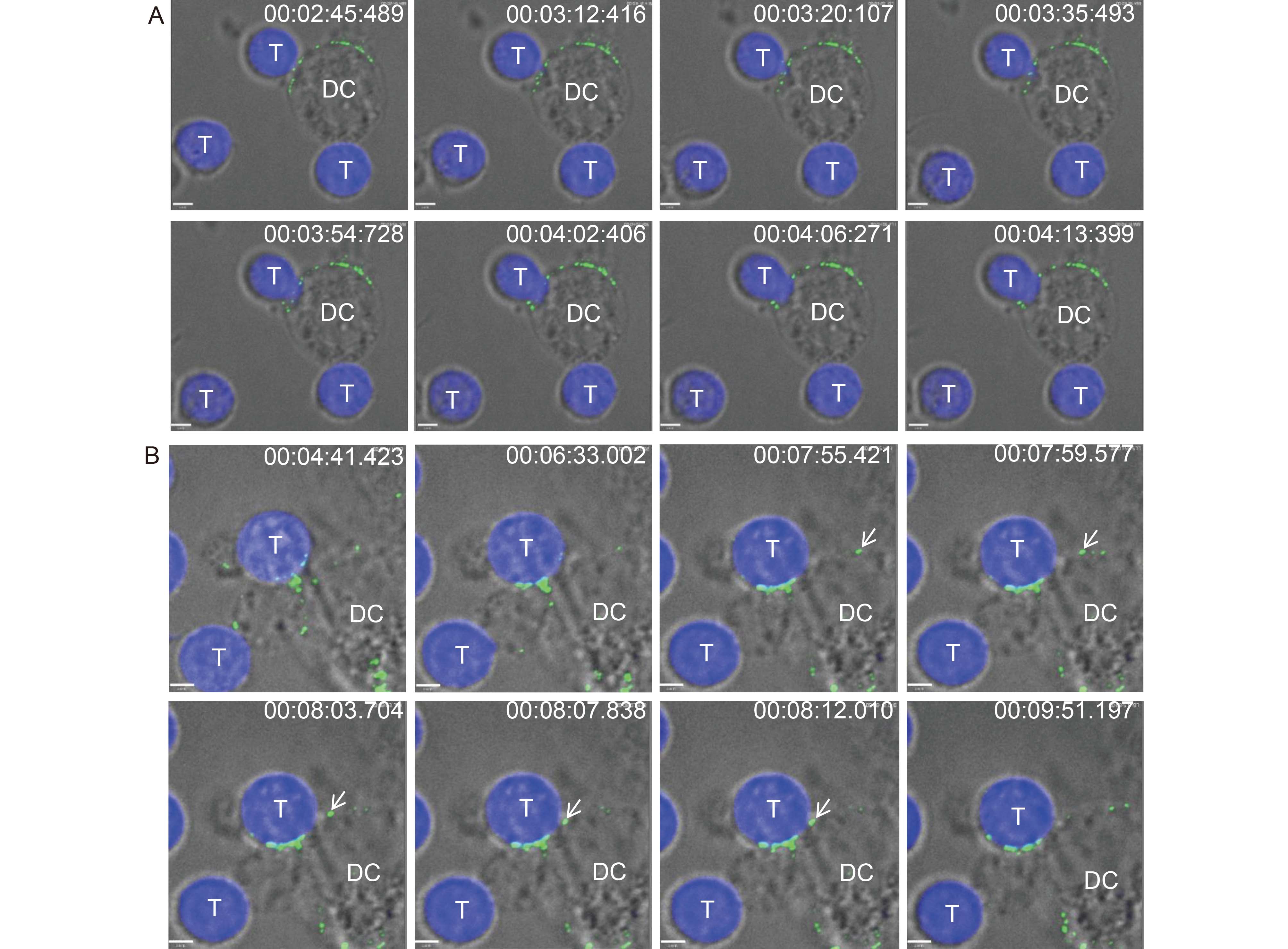
Figure 3. Live cell images of JEV particles transmitted from DCs to T cells. CellTracker Blue-labeled T cells were added to DCs (pulsed with JEV-AF488 on ice for 1 h) before analysis by live-cell confocal microscopy at 37 °C. (A) The processes of JEV transmission from DCs to T cells were: T cells “scanned” DCs followed by the formation of junctions. (B) Transfer of viral particles from DCs to T cells. Arrows indicate typical viral particles. Scale bars, 3.3 μm (A) and 2.9 μm (B). Results of a representative experiment (n = 3) are shown.
-
The antigen-presenting and migratory capabilities of DCs are exploited by viruses such as HIV-1 to invade immune surveillance and gain access to cells that are more permissive for viral replication (Geijtenbeek et al., 2000a; Norcross, 1984). Although DC-SIGN has been shown to bind many viruses, its role in mediating trans-infection of T cells has only been described for HIV-1, cytomegalovirus, enterovirus, and measles virus (Geijtenbeek et al., 2000b; de Witte et al., 2008; Halary et al., 2002; Ren et al., 2014). The findings presented here expand these observations to a flavivirus: DC-SIGN facilitated JEV transmission from DCs to T cells in trans, and DC-captured JEV particles were transferred to T lymphocytes via VS formed at DC-T cell junctions.
Studies on HIV-1 have shown that DC-SIGN promotes the formation of VS between HIV-1-pulsed DCs and CD4+ T cells, allowing HIV-1 to escape immune surveillance before dissemination to CD4+ T cells. DC-SIGN-bound HIV-1 particles are stable and retain their infectivity for days, which is important for long-term HIV-1 transmission. Our live-cell imaging study revealed that VS formation was initiated by normal cellular interactions in which T cells randomly “scanned” DCs until a stable DC-T cell junction was formed. JEV particles captured by DCs were concentrated at the VS before their transfer. The VS formed rapidly within a few minutes after the incubation of JEV-pulsed DCs with T cells and then underwent dissociation, and most viral particles were internalized into DCs within 15 min. An open question is whether viral internalization is a prerequisite for JEV transmission from DCs to T cells in trans.
Further to our initial observations that DC-SIGN efficiently captures and concentrates viral particles at the cell surface to facilitate the cis-infection of JEV (Wang et al., 2016), the results of the current study indicate that JEV is also transferred from DCs to T cells via VS formed at DC-T cell junctions. This suggests that DC-SIGN is a potential therapeutic target for treatment of JEV infection. It will be important to determine in future studies whether DC-SIGN-mediated JEV transmission occurs in vivo, establish the degree to which this is responsible for the ability of DCs to potentiate T cell infection, and evaluate how this affects JEV pathogenesis. JEV can replicate in various types of primary cells and immortalized cell lines from a wide variety of avian, mammalian, amphibian and insect species, suggesting that multiple receptors are likely to be involved in JEV infection (Pierson et al., 2013). In our study, mAb161 resulted in partial inhibition. Although we used relatively high dose of mAb161, the inhibitory effect did not improve, suggesting that other potential molecules likely also contribute to JEV binding/entry. Given that JEV did not bind efficiently to T cells in the absence of DCs, we speculate that JEV binding to DC-SIGN induces a conformational change that promotes a closer interaction between E protein and T cells. Alternatively, the binding of JEV particles to DC-SIGN may concentrate virions at the surface of DCs and thus increase the probability that other cells in contact with DCs become infected.
-
This work was supported by the National Key Research and Development Program of China (2016YFC1200400), the National Natural Science Foundation of China Grants (81572009 and 31570165) and the National High Technology Research and Development Program of China (2014AA021406). We thank the Core Facility and Technical Support at Wuhan Institute of Virology for technique supports of Confocal Microscopy (Dr. Ding Gao) and Flow Cytometry (Ms. Juan Min).
-
The authors declare that they have no competing interests. All protocols involving human blood samples were reviewed and approved by the Local Research Ethics Committee. Informed written consents from the human subjects were obtained in this study.
-
PW performed the major experiments and statistical analysis, drafted and revised the manuscript. ML, WL and DZ participated in the statistical analysis and revised the manuscript. QXH and YLL designed the study, drafted and revised the manuscript. All authors read and approved the final manuscript.
Supplementary materials are available on the websites of Virologica Sinica: www.virosin.org; link.springer.com/journal/12250.
-
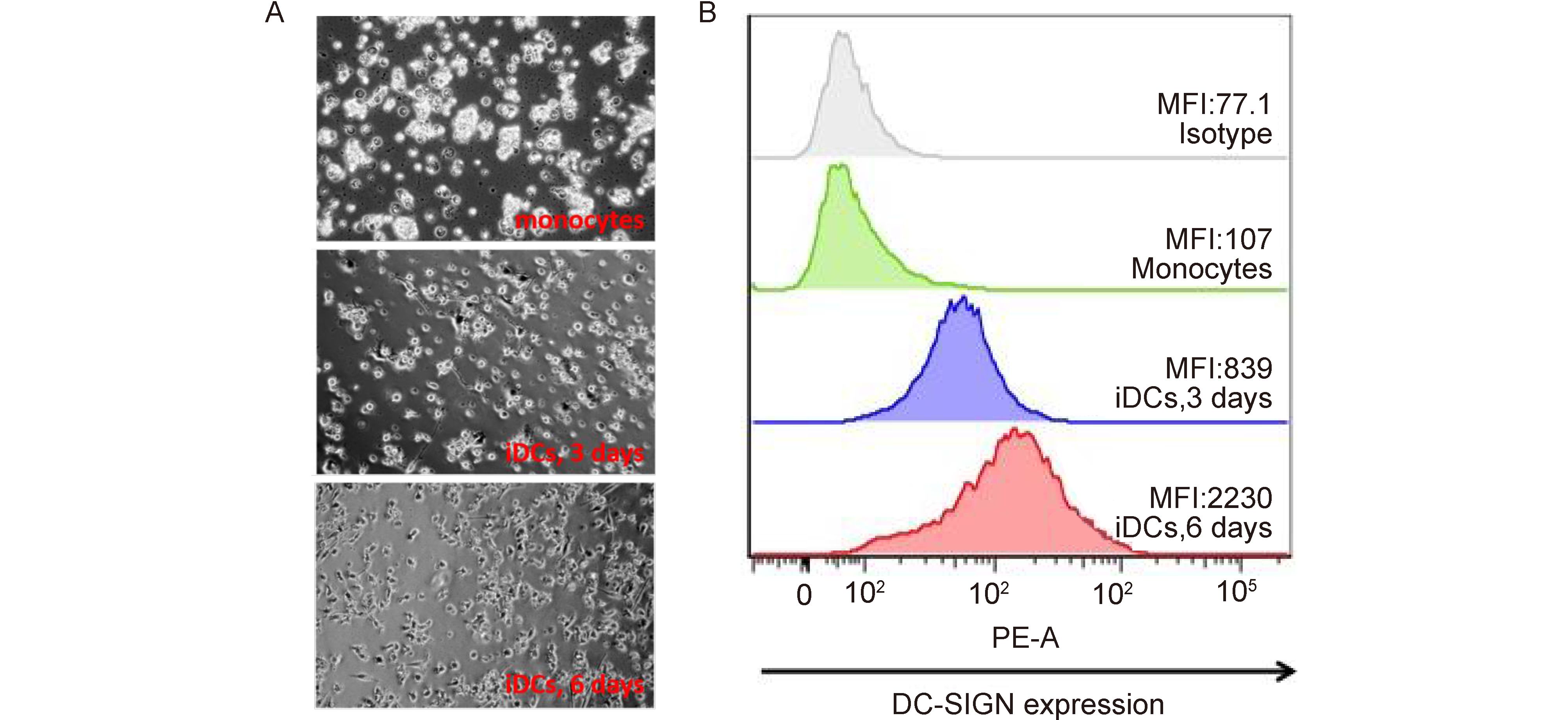
Figure Material S1. DCs and DC-SIGN expression. (A) DC morphology. (B) The surface expression of DC-SIGN on DC
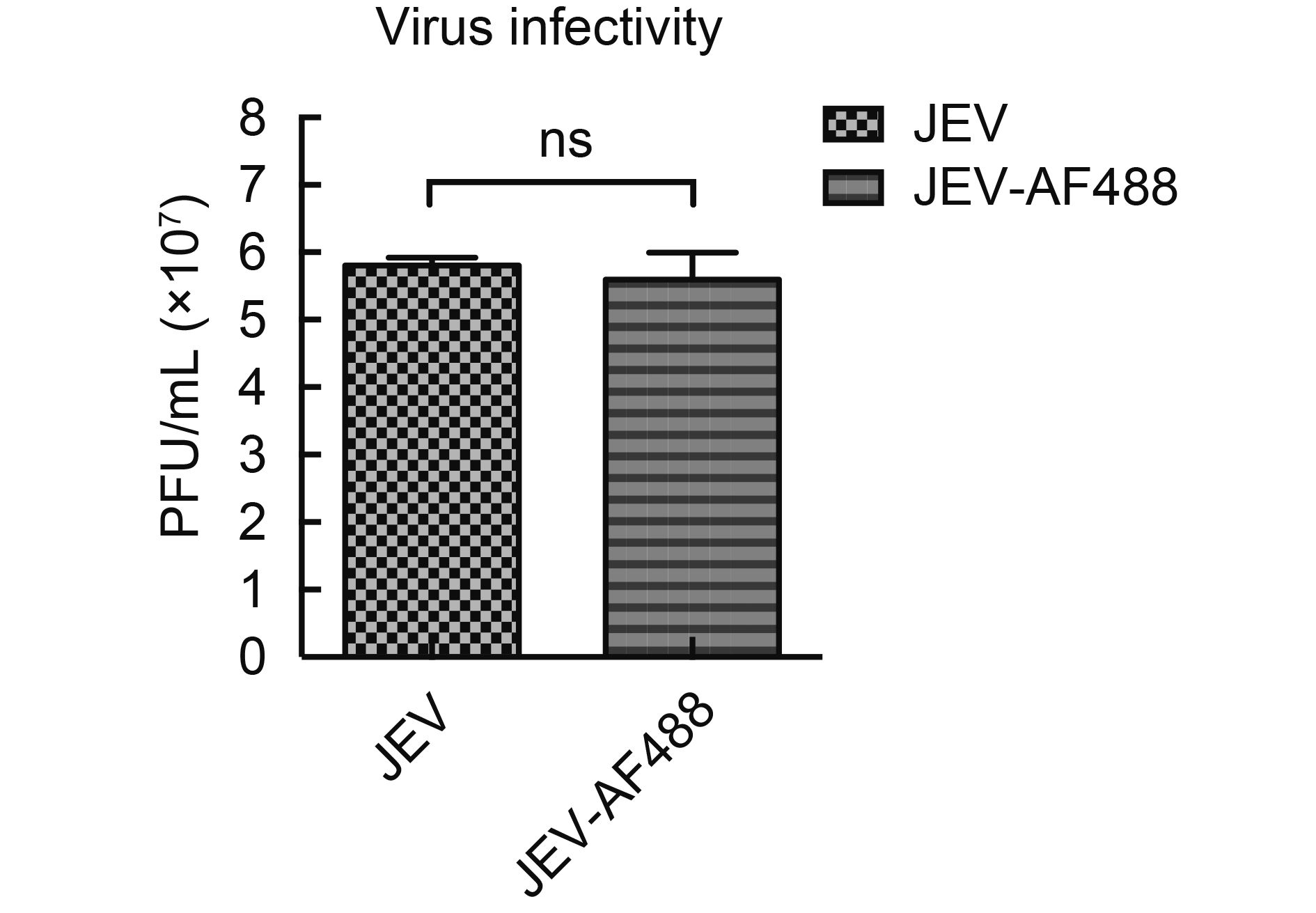
Figure Material S2. Infectivity of JEV and JEV-AF488. JEV or JEV-AF488 (MOI = 0.1) were used to infect Raji-DCSIGN cells for 3 days. The supernatants were collected and titered in BHK-21 cells. Data represent mean ± SD of three independent experiments. Means were compared with the Student’s t-test. ns, Non-significant
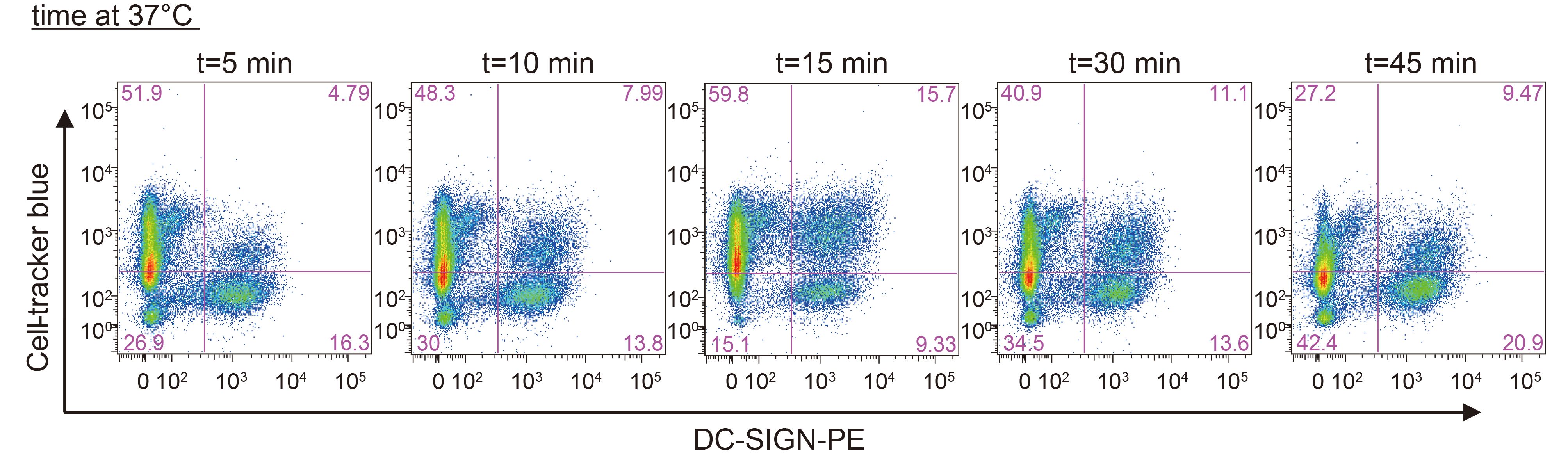
Figure Material S3. Formation of DC-T clusters. DCs were pulsed with JEV-AF488 (MOI = 10) on ice for 1 h, washed extensively to remove unbound viruses and then incubated with CellTracker Blue-labeled T cells for the indicated times before fixation and staining with phycoerythrin (PE)-conjugated DC-SIGN (DC-SIGN-PE). CD-T clusters were first gated on JEV-AF488+ populations followed by analysis with CellTracker Blue+ PE+ cells. Results of a representative experiment (n = 3) are shown
Material S4. The VS between DCs and T cells formed in a few minutes (Download to play the AVI movie).
Material S5. JEV viral particles moved to the VS and were then directionally transferred to T cells (Download to play the AVI movie).
DC-SIGN promotes Japanese encephalitis virus transmission from dendritic cells to T cells via virological synapses
- Received Date: 14 June 2017
- Accepted Date: 18 August 2017
- Published Date: 31 August 2017
Abstract: Skin-resident dendritic cells (DCs) likely encounter incoming viruses in the first place, and their migration to lymph nodes following virus capture may promote viral replication. However, the molecular mechanisms underlying these processes remain unclear. In the present study, we found that compared to cell-free viruses, DC-bound viruses showed enhanced capture of JEV by T cells. Additionally, JEV infection was increased by co-culturing DCs and T cells. Blocking the C-type lectin receptor DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) with neutralizing antibodies or antagonists blocked JEV transmission to T cells. Live-cell imaging revealed that DCs captured and transferred JEV viral particles to T cells via virological synapses formed at DC-T cell junctions. These findings indicate that DC-SIGN plays an important role in JEV transmission from DCs to T cells and provide insight into how JEV exploits the migratory and antigen-presenting capabilities of DCs to gain access to lymph nodes for dissemination and persistence in the host.
















 DownLoad:
DownLoad: