HTML
-
Rabies, which is one of the oldest infectious diseases and is caused by the RABV, still poses a threat to public health throughout the world (Zhao et al. 2010; WHO 2013; Molanouri Shamsi et al. 2017). Over 3 billion humans are threatened by rabies, and there are more than 59, 000 deaths every year, most of which occur in developing countries within Asia and Africa (Fooks et al. 2014; Chen et al. 2015). Vaccination is the most effective way to prevent and control rabies. China, which is the biggest market for human rabies vaccine consumption, commits a substantial amount of money and personnel into the control of rabies each year (Chen et al. 2015).
It has been reported that the functions of several components of both the innate (e.g., natural killer cell activity and neutrophil oxidative burst activity) and adaptive (e.g., T and B cell functions) immune system could be suppressed after exhaustive exercise (Nieman 1997; Steensberg et al. 2001; Szymura et al. 2016; Liu et al. 2017; Zamani et al. 2017). Even a single acute bout of exercise is reported to elicit significant changes in the immune system (Walsh et al. 2011). Other studies demonstrated that moderate exercise as a behavioral factor could be helpful for controlling sickness, infections and tumors, as well as enhancing the immune response (Nieman et al. 1990; Hojman 2017; Molanouri Shamsi et al. 2017). However, it is still not clear whether involvement in exhaustive exercise will affect the immune response after rabies vaccination.
In the present study, the relationship between exhaustive exercise (swimming was specified in this study) and the humoral immune response induced by the rabies vaccine was investigated in a mouse model of one-time exhaustive exercise. It was found that there was no significant difference on body-weight changes, VNA titers, antibody subtypes and survival ratio between the mock group and the exhaustive group after rabies vaccination.
-
BSR cells, a cloned cell line derived from BHK-21 cells, were maintained in Dulbecco's modified Eagle's medium (DMEM, Mediatech, Herndon, VA) containing 10% FBS. RABV LBNSE was propagated in BSR cells. LBNSE, an attenuated vaccine strain, was constructed from the SADB19 strain through a mutation of the G at amino acid positions 194 and 333 (Rasalingam et al. 2005; Wen et al. 2011). LBNSE were UV-inactivated with a 254-nm UV light source for 30 min before immunization. The challenge virus CVS-24 was propagated in the brains of suckling mice. A fluorescein isothiocyanate (FITC)-conjugated antibody against RABV N was purchased from Fujirebio (Malvern, PA). The antibodies used for ELISA analyses including HRP-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b and IgM, were all purchased from BioLegend (San Diego, CA). Eight-week-old female ICR mice and 1-day-old suckling Kunming mice were purchased from the Center for Disease Control and Prevention of Hubei Province, China. The mice were housed in a controlled specific pathogen-free environment at room temperature (22 ± 2 ℃) and moderate humidity (45%– 55%). All animal experiments were carried out as approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2015-016).
-
A mouse model of one-time exhaustive exercise was established firstly. Eight-week-old female ICR mice were allowed to adapt to their surroundings for 1 week before being subjected to the experiments, and all of the mice were adapted to water for 3 days before the formal experiment. Then those mice were randomly divided into four groups (n = 5) and were subjected to a forced-swimming exercise (an artificial flow was created with a magnetic stirrer) in a round container (diameter: 45 cm and depth: 35 cm) filled with water at 30 ± 2 ℃. This device enabled the swimming evaluation of 20 mice simultaneously. It has been demonstrated that the intensity of swimming exercise was significantly raised by interaction among the mice (Iemitsu et al. 2004). The water depth was 35 cm so that the mice could not support themselves by touching the bottom with their feet (Mazzardo-Martins et al. 2010). The swimming was continuously monitored. GLU, LAC, UREA, CK, SOD and MDA in the sera were measured at 0, 45, 90 and 120 min by ELISA. After swimming, the mice were dried with a towel and kept warm by an electric heater. The exhaustion standard involved sinking to the bottom of the water for no less than 3 s and the inability to respond to outside stimuli.
As shown in Fig. 1, the mice immunized with inactivated LBNSE by the intramuscular (i.m.) route immediately followed by 45 or 90 min of swimming or no swimming were designated LBNSE + 45 min swimming (aerobic exercise group), LBNSE + 90 min swimming (exhaustive exercise group) and LBNSE, respectively. The forced swimming was carried out daily for 2 weeks in the swimming groups. Body weight of all mice were weighed daily for 2 weeks. Additionally, the mice in the mock group immunized with the same volume (100 μL) of DMEM with 90 min of swimming were designated DMEM. The swimming was conducted at 30 ± 2 ℃ water, and the mice body weights were measured daily for 2 weeks. VNA titers and antibody subclasses in the sera were evaluated every week. After 4 weeks post immunization (wpi), the mice were challenged with 50 times of the mouse intracranial median lethal dose (MICLD50) of CVS-24 by the intracranial (i.c.) route under conditions of isoflurane anesthesia. The insulin syringe with 29G × 1/2" guage (0.33 mm × 12.7 mm) was used when i.c. challenge and the volume of inoculum was 30 μL. All the mice were then observed for 2 weeks for clinical signs of rabies. The mice that appeared moribund or that lost more than 30% of their body-weight were humanely euthanized with aether.
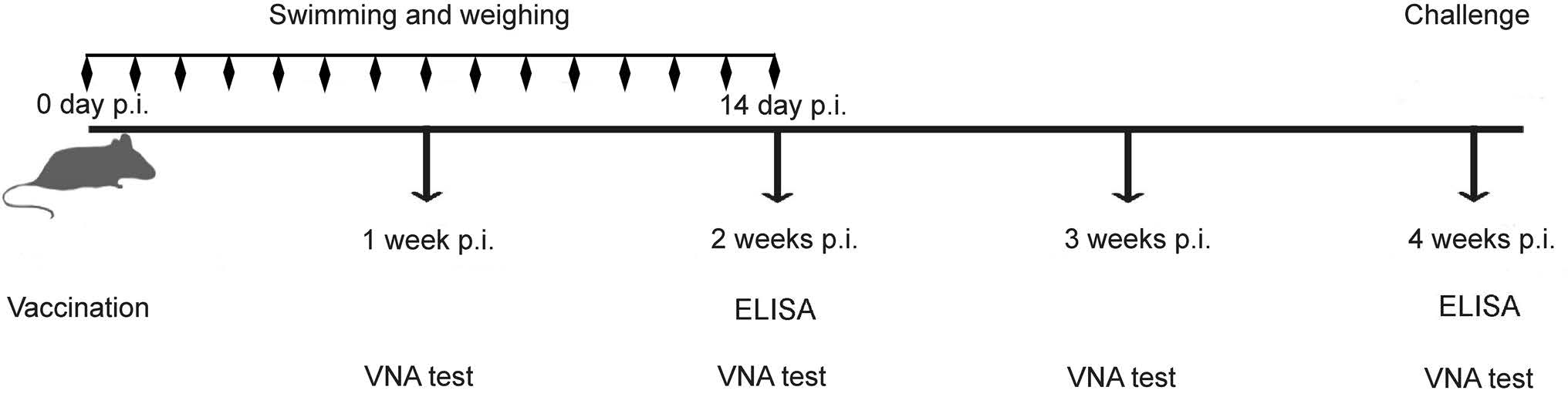
Figure 1. Schematic of the experimental design. Eight-week-old female ICR mice (n = 15) were immunized by the i.m. route with 107 FFU of LBNSE or mock-immunized with DMEM. The blood samples were collected and separated sera was used to test VNA by FAVN and the immunoglobulin subclass antibody by ELISA at each time point as described in the text. At 4 wpi, the mice were challenged by the i.c. route with 50 MICLD50 of CVS-24.
-
Blood samples (100 μL) were collected and mixed with an equal volume of 7.5% EDTA-Na2 at 0, 45, 90 and 120 min to analyze the level of the important indicators of sports fatigue [glucose (GLU), lactic acid (LAC) and urea (UREA)] and the indicators of oxidative tissue damage [creatine kinase (CK), superoxide dismutase (SOD) and malondialdehyde (MDA)]. After centrifugation for 10 min at 10, 000 ×g, the supernatant was recovered. The GLU, LAC, UREA and CK were determined using a IDEXX biochemical analyzer, the SOD and the MDA were analyzed by the Hydroxylamine method and colorimetry, respectively, according to the manufacture's (Nanjing Jiancheng Bioengineering Institute) instruction.
-
VNA titers were measured by an OIE prescribed method, fluorescent-antibody virus neutralization (FAVN) test, described elsewhere (Cliquet et al. 1998). Briefly, 50 μL volumes of serial threefold dilutions of test sera and standard serum were prepared on 96-well microplates in 150 μL volumes. Each sample was added to four adjacent wells. A 50 μL volume of a rabies challenge virus (CVS-11) suspension, containing 50–200 fluorescent focus units (FFU), was added to each well. The microplates were then incubated at 37 ℃ for 1 h in an incubator with 5% CO2. Following incubation, 50 μL of the cell suspension, containing 2 × 104 BSR cells, was added to each well, and the microplates were incubated at 34 ℃ in an incubator with 5% CO2 for 60 h. Then the plates were fixed with 80% icecold acetone at 20 ℃ for 30 min and air dried. Cells were stained with FITC-conjugated antibodies against RABV N for 45 min at 37 ℃. After staining, cells were washed three times with phosphate-buffered saline (PBS). The results were assessed by using an Olympus IX51 fluorescence microscope. The neutralizing antibody titers were calculated by Spearman-Kärber formula. VNA titers were expressed in international units per milliliter by comparison with the titer of a reference serum (obtained from the National Institute for Biological Standards and Control, Herts, United Kingdom) included in each test.
-
ELISA plates were coated with coating buffer (5 mmol/L Na2CO3, pH 9.6) containing 500 ng/well of purified RABV overnight at 4 ℃. The plates were then washed three times in PBS and blocked in 5% low-fat milk-PBS for 2 h at 37 ℃. The sera were diluted 30 times in 100 μL PBS, and then added to the RABV G-containing wells. The samples were incubated for 2 h at 37 ℃. After incubation, the plates were washed three times with PBS, and then 100 μL of horseradish peroxidase (HRP)-conjugated goat antimouse IgG (1:1000), IgG1 (1:1500), IgG2a (1:1500), IgG2b (1:2000), IgG3 (1:1500) or IgM (1:2000) (Boster, Wuhan, China) was added to each well, respectively. The plates were incubated at 37 ℃ for 45 min, and then washed three times with PBS-Tween. Tetra-methyl-benzidine (TMB) substrate was then prepared and added to each well according to the instructions of the manufacturer (Boster, Wuhan, China). The plates were incubated for 30 min at 37 ℃ in the dark, and the reaction was stopped by adding 2 M H2SO4. Optical density was read at 450 nm using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA).
-
All of the data were analyzed with GraphPad Prism software (GraphPad Software, Inc., CA). An unpaired twotailed t test was used to determine the statistical significance of the contents of the GLU, LAC, UREA, CK, SOD and MDA, and the VNA titers. For the results of survivorship after the challenge, Kaplan-Meier survival curves were analyzed by the log-rank test. For all of the tests, the following notations were used to indicate the significant difference: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Cells, Viruses, Antibodies, and Animals
Experimental Design
Measurement of of GLU, LAC, UREA, CK, SOD and MDA in the Sera
VNA Test
Evaluation of RABV-Specific Antibody Subtypes and IgG Subclasses by ELISA
Statistical Analysis
-
ICR mice were used to create an animal model of one-time exhaustive exercise. All of the mice were adapted to water for 3 days before the formal experiment. The adaptation was aimed to reduce the water-induced stress without promoting physiological alterations in relation to the formal experiment (Contarteze et al. 2008). The adaptation consisted of keeping the mice in shallow water at 30 ± 2 ℃ (Harri and Kuusela 1986) for 7 days and 60 min/day. All of the mice were immediately euthanized with aether after exhaustive exercise.
To evaluate when the mice reach the exhaustive condition after acute swimming, the sera were collected at the indicated time points (0, 45, 90 and 120 min) and immediately evaluated by the biochemical analyzer, hydroxylamine method or colorimetry method. It was found that the concentrations of UREA, CK and MDA significantly increased at 90 min compared with their initial value (Fig. 2B, 2E, 2F, respectively). The LAC and MDA in the sera attained a peak value (1.44 mmol/L and 43.83 nmol/mL, respectively, Fig. 2C, 2F) at 90 min, and the concentrations of UREA and CK consistently increased over time (Fig. 2B, 2E, respectively). However, as depicted in Fig. 2A and 2D, the content of GLU and SOD in the sera significantly decreased at 90 min compared with their initial value. We also found that the first mouse showed exhaustion signs after 82 min. Taken together in this study, 90 min of swimming was considered exhaustive exercise.
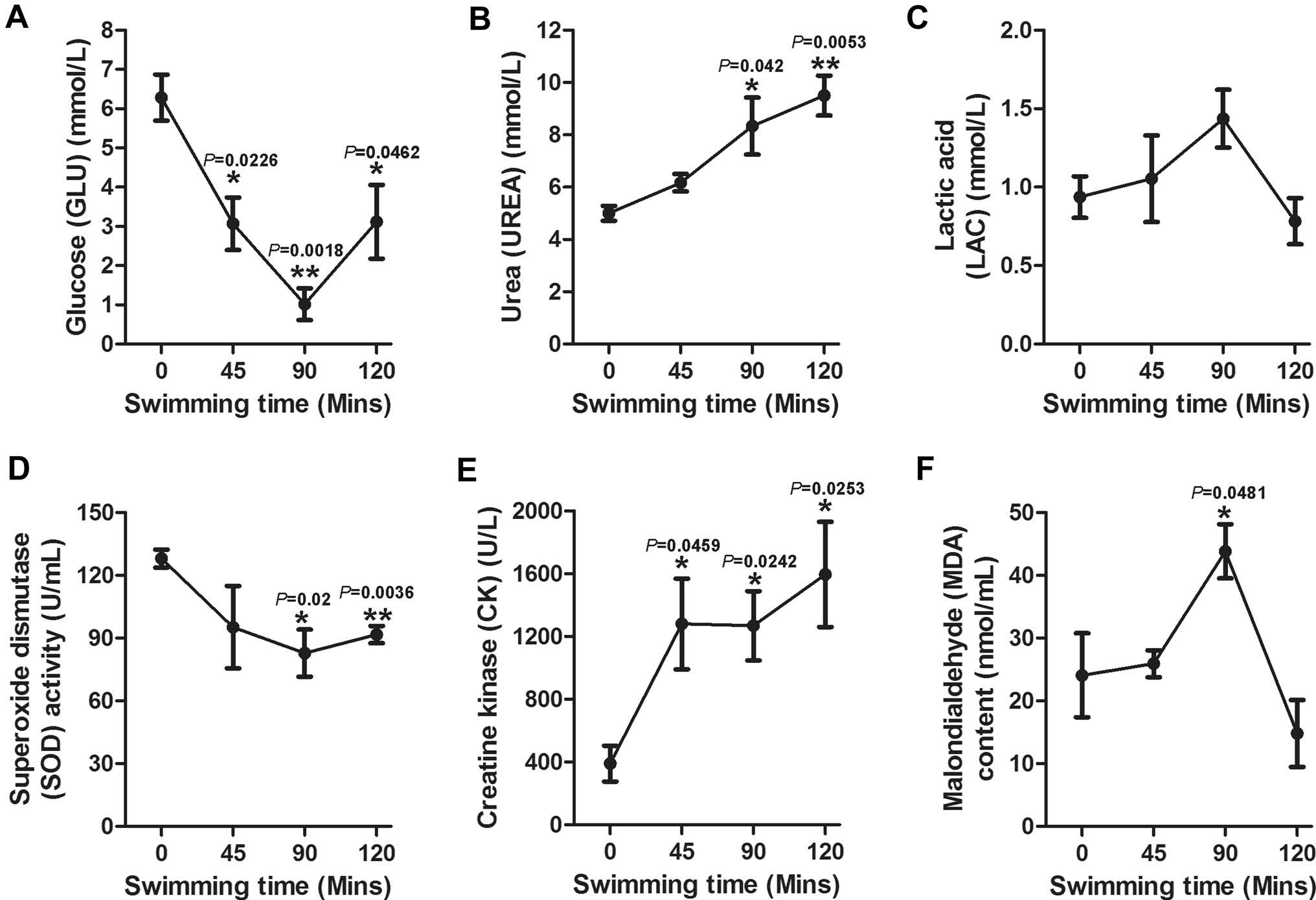
Figure 2. Concentrations of GLU, VREA, LAC, SOD, CK and MDA in mice sera during swimming. The sera were collected to test UREA, CK, GLU and LAC by a biochemical analyzer, and analysis of SOD and MDA was conducted by the hydroxylamine and colorimetry method, respectively, at the indicated time points (0, 45, 90 and 120 min). The results are presented as the mean ± SE (n = 5). Asterisks (*P < 0.05; **P < 0.01; ***P < 0.001) indicate significant differences among the different time points as analyzed by the unpaired t test.
-
Prior to checking the effect of exhaustive exercise on the humoral immune response, the body-weight changes and the level of fatigue of mice under conditions of different exercise intensity were monitored. Very few mice sunk to the bottom of the water within 90 min in the first 5 days, all other mice in DMEM + 90 min swimming group and LBNSE + 90 min swimming group showed inability to respond to outside stimuli after 90 min swimming. As depicted in Fig. 3, no significant difference was observed in mice among any of the groups; this outcome indicated that the mice could rapidly recover after exhuastive exercise daily for 2 weeks.
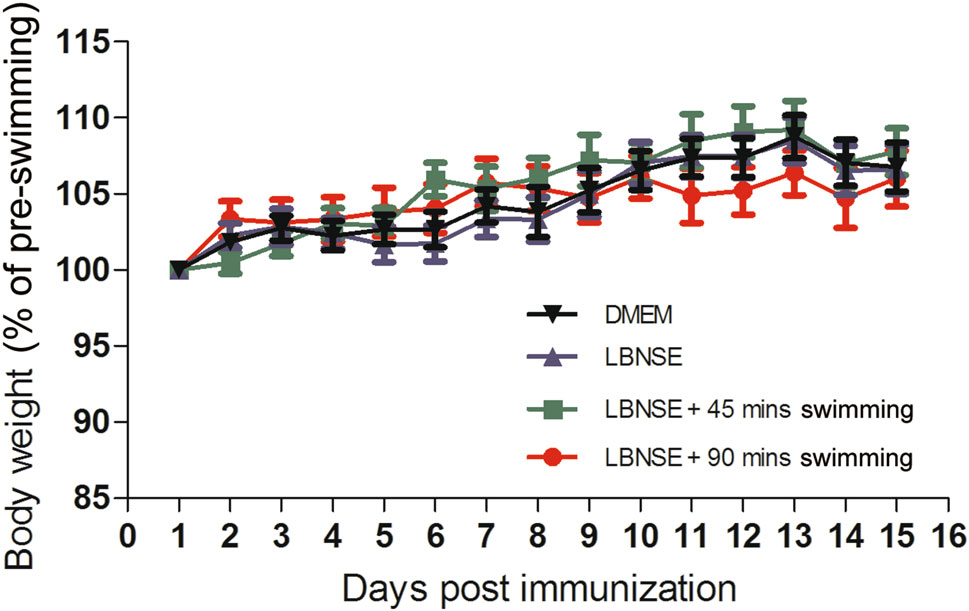
Figure 3. The body-weight change of immunized mice with a different intensity of exercise. Eight-week-old female ICR mice (n = 15) were immunized by the i.m. route with 107 FFU of LBNSE or mock immunized with the same volume of DMEM and trained to swim for 45 or 90 min during the first 2 weeks after immunization. The bodyweights were measured daily and the curves for the body-weight changes were depicted accordingly.
-
To determine whether exhaustive exercise could affect the humoral immune responses after rabies vaccination, the mice were immunized with 107 FFU of LBNSE or the same volume of DMEM, and this immunization was followed by 45 or 90 min of swimming or no swimming in the different experimental groups as described in the "Experimental Design." The blood samples were collected weekly from 1 to 4 wpi, and the sera were used for the VNA titration via FAVN tests and the testing of antibody subtypes. As shown in Fig. 4A, no significant difference of the VNA titers was observed among any the LBNSE immunized mice. These data indicate that exhaustive exercise does not affect the VNA production after rabies vaccination in mice.
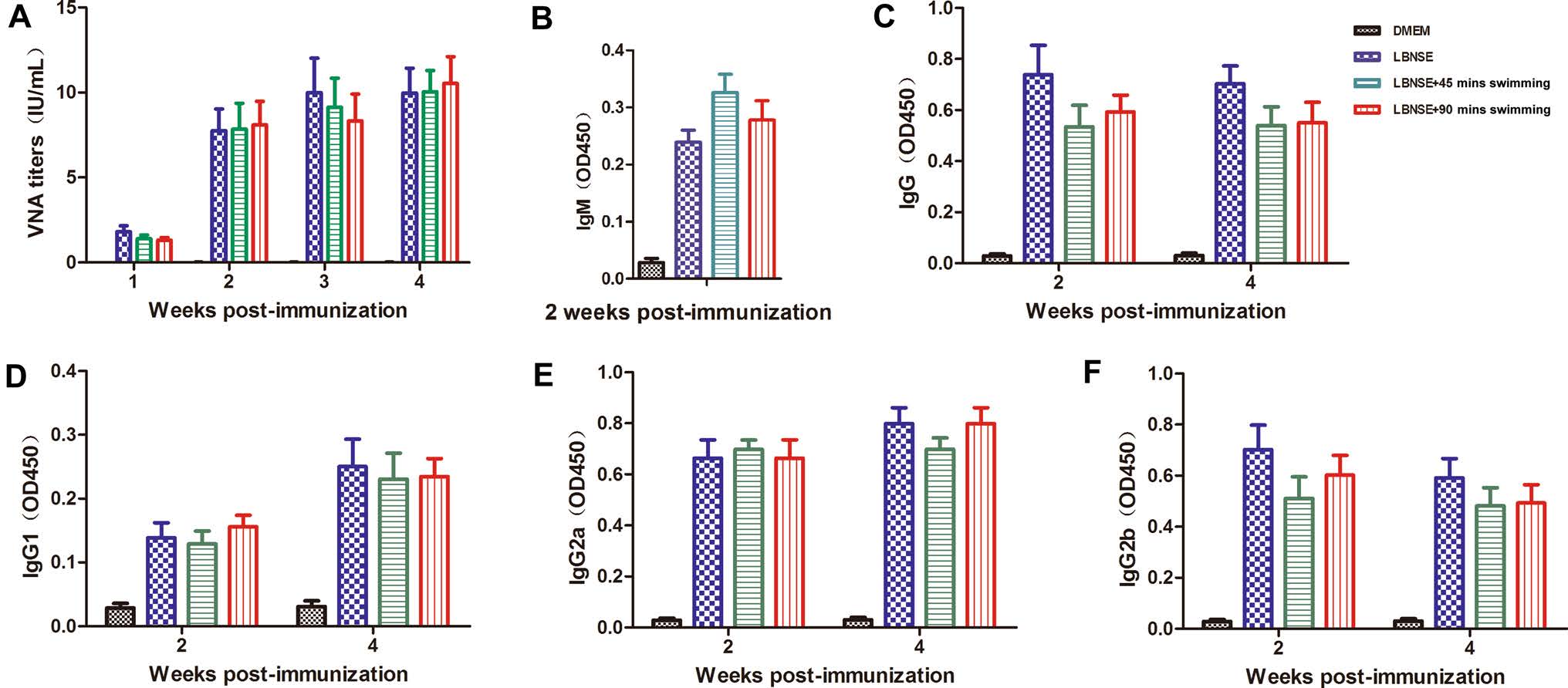
Figure 4. Exhaustive exercise after rabies immunization does not affect the VNA titers and antibody subtype production in mice. Eight-weekold female ICR mice (n = 15) were i.m. immunized with 107 FFU of LBNSE or mock immunized with the same volume of DMEM. The mice of the exercise groups were trained to swim for 45 or 90 min during the first 2 weeks after immunization. At the indicated time points post immunization, the sera were collected to determine the VNA titers (A). Optical density (OD) values of total IgM (B), IgG (C), IgG1 (D), IgG2a (E), and IgG2b (F) against RABV were then determined by ELISA.
In addition, the antibody subtypes (IgM and IgG) and IgG subclasses (IgG1, IgG2a and IgG2b) in sera at 2 and 4 wpi were quantified by ELISA. As shown in Fig. 4B, 4C, no significant difference was observed at 2 or 4 wpi for the total IgM and IgG. It was reported that acute exercise may affect immune cell activity through muscle contractioninduced release of immune regulatory cytokines (Catoire and Kersten 2015). Many of these cytokines are also recognized for their role in regulating immune cells. IgG1 is associated with the stimulation of Th2-type immune responses that can regulate the humoral immune responses (Ozaki et al. 2002). IgG2a and IgG2b, stimulated during Th1-type immune responses, were suggested to be the main form of immunoglobulin induced by rabies vaccines (Singh and O'Hagan 1999). As shown in Fig. 4D–F, no significant difference were detected in mice immunized with LBNSE for IgG1, IgG2a and IgG2b levels at both 2 and 4 wpi. These results suggest that exhaustive exercise does not affect the RABV-specific antibody subtypes or IgG subclasses after rabies vaccination in mice.
-
To investigate whether the protection rate would be affected by exhaustive exercise after rabies vaccination, the mice were challenged by i.c. with 50 MICLD50 of CVS-24 at 4 wpi. The clinical symptoms of rabies were scored and the numbers of deaths were recorded daily for 2 weeks after the challenge. As shown in Fig. 5A, severe clinical signs were observed in the mice of the mock group (DMEM + 90 min swimming) as early as 6 days post challenge. In addition, all of the mice in the mock group succumbed to rabies within 12 days. Furthermore, the death started at 11, 9, and 10 days post challenge for the mice in the LBNSE group, the LBNSE + 45 min swimming group and the LBNSE + 90 min swimming group, respectively (Fig. 5B–5D). The difference of the protection rate between the LBNSE group (37.5%) and the LBNSE + 90 min swimming group (33%) was not significant. Whereas, there was a very significant difference (P < 0.001) in protection rate between mock group and LBNSE group (Fig. 5E). These results are consistent with the VNA titers induced in the immunized mice. Collectively, the exhaustive exercise after rabies vaccination did not affect the immune responses of the immunized mice.
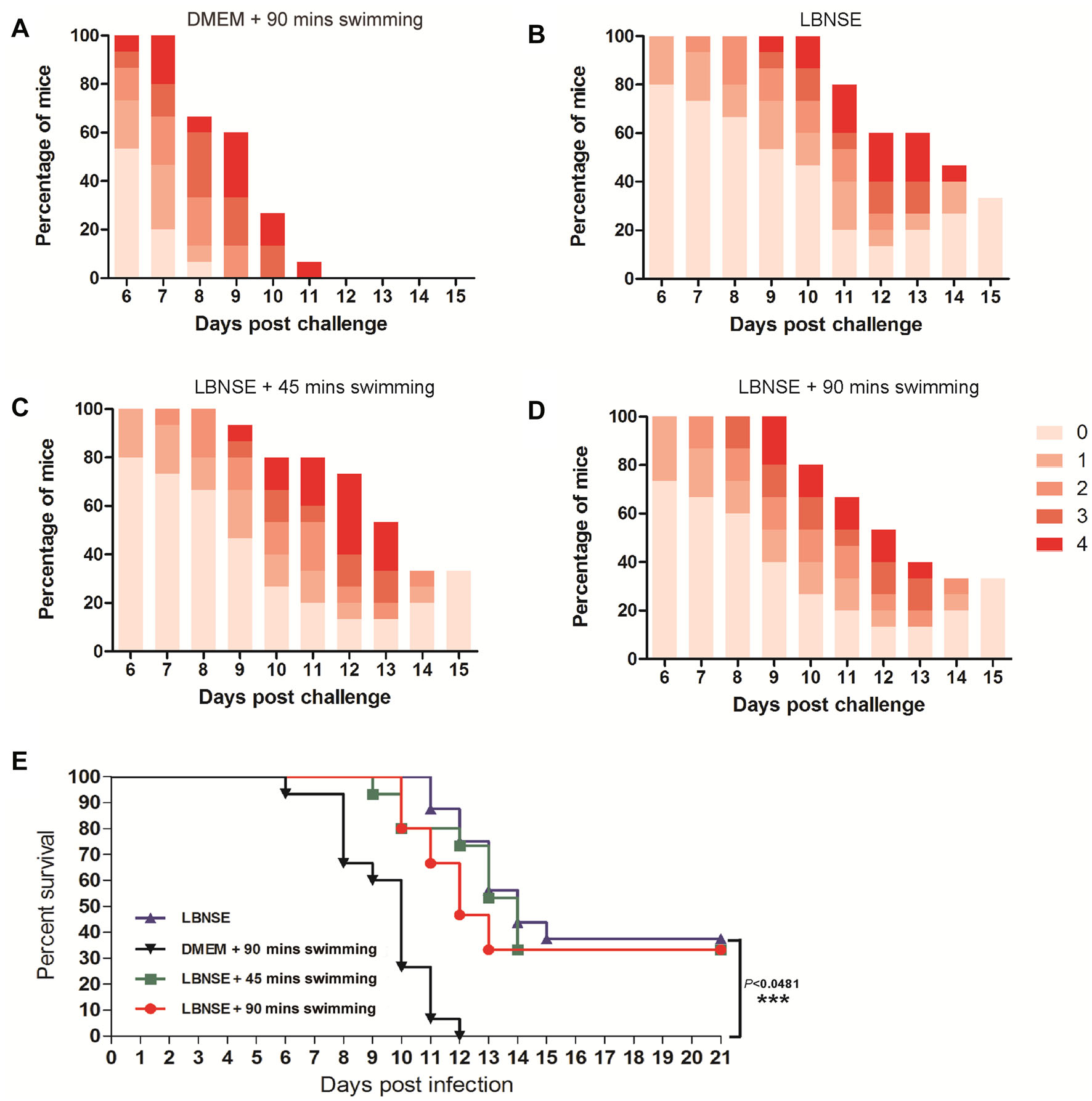
Figure 5. Protection of immunized mice with exhaustive exercise after challenge. After 4 weeks post vaccination, the immunized mice were i.c. challenged with 50 MICLD50 CVS-24 and observed daily for 2 weeks. The number of survivors and clinical signs in each group was recorded. The clinical score (A–D) was monitored and calculated according to the following clinical signs: 0, normal mouse; 1, disorder movement; 2, ruffled fur; 3, trembling and shaking; 4, paralysis; and 5, dead. The survival ratio (E) was analyzed according to the numbers of mice.
The Establishment of the Mouse Model of OneTime Exhaustive Exercise
Body-Weight Changes of Mice Under the Exercises of Different Intensity
VNA Induction and RABV-Specific Antibody Isotype Identification in Immunized Mice with Exhaustive Exercise
Protection of Immunized Mice with Exhaustive Exercise after a Lethal Challenge
-
The most important reason why people abuse the rabies vaccine is because of fears of the protective effect of the rabies vaccine. There is no doubt that a healthy diet, a good lifestyle and adequate rest benefit human immunity. Moderate and regular exercise has been demonstrated to play a key role in increasing resistance to common infectious diseases and in reducing the risk of developing diseases (Baynard et al. 2012; Potenza et al. 2016). However, exhaustive exercise is known to induce a situation of oxidative stress, fatigue and tissue injury (Malaguti et al. 2009). There is no clear evidence that exhaustive exercise after immunization would affect the effectiveness of the rabies vaccine.
Several studies have reported that a mouse or rat model revealed similarities in the adaptations to exercise in relation to those observed in humans. As an innate ability of rodents, swimming exercise presents advantages over treadmill running (Alexandre et al. 2001; Seo et al. 2014). Previous studies suggest that long-term strenuous exercise could cause less GLU, more LAC and more UREA in the sera (Terada et al. 2001). Therefore, the level of GLU, LAC or UREA in the sera correlates with the intensity of the exercise. CK, MDA and SOD are important tissue damage markers (Jia et al. 2017). In our study, the changes in these indicators (GL, LAC, UREA, CK, MDA and SOD) are consistent with previous studies. The exhaustive signs were gradually observed after 82 min-swimming. Together, these data illustrate that the construction of the mouse model for one-time exhaustive exercise was successful in this study.
T cells play a very important role in controlling virus infection. Nevertheless, mice lacking CD8+T cells showed no significant difference in the development of clinical signs after RABV strain CVS-F3 infection by comparison with intact counterparts with the same genetic background (Hooper et al. 1998). In other words, the humoral immune response rather than the cellular immune response is crucial for the control of rabies. Therefore, in this study, the effects of exhaustive exercise on T cell responses were not evaluated.
In this study, we proved for the first time that exhaustive exercise does not affect antibody responses and protection after rabies immunization. In addition, our research may be more informative to evaluate the effects of exhaustive exercise on the immune responses prior to rabies immunization and we will continually focus on this in our future study.
In summary, exhaustive exercise after rabies vaccination did not affect the VNA titers, antibody subtypes and survivorship after the RABV challenge in mice. These findings provide a new understanding of the relationship between exhaustive exercise and the effectiveness of the rabies vaccine.
-
This work was partially supported by the National Natural Science Foundation of China (31702248, 31402176, 31372419 and 31522057), the National Science Foundation for Postdoctoral Scientists of China (Grant No. 20163M590701), the National Program on Key Research Project of China (2016YFD0500400), the Fundamental Research Funds for the Central Universities (2662016QD036, to MZ) and the Ministry of Agriculture of China (special fund for Agro-scientific research in the Public Interest, 201303042).
-
ZFF, LZ, MZ, DYT conceived and designed the experiments. LX, MRL, YJ Z, JCR, JP, JLS performed the experiments. DYT, LZ, MZ analyzed the data. LX, DYT, MZ wrote the manuscript and prepared the Figures. ZFF, LZ, MZ, DYT checked and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Author Contributions
-
The authors declare that they have no conflict of interest.
-
Animal experiments in this study were approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2016-047).














 DownLoad:
DownLoad: