HTML
-
Severe fever with thrombocytopenia syndrome virus (SFTSV), a tick-borne pathogen (Yu et al. 2011), belongs to the genus Phlebovirus of the family Phenuiviridae (Saijo 2018). SFTSV consists of three single-strand negativesense RNA segments that include the large segment (L), encoding the RNA-dependent RNA polymerase (RdRp), the medium segment (M), encoding the virus envelope glycoproteins consisting of glycoprotein N (Gn) and glycoprotein C (Gc), and the small segment (S), encoding the viral non-structural proteins (NSs) and the N protein (NP) (Yu et al. 2011; Lei et al. 2015).
NSs is the main virulence factor of SFTSV, which also plays an important role in the regulation of innate immune responses (Qu et al. 2012; Santiago et al. 2014; Ning et al. 2015). Qu et al. (2012) have reported that the production of interferon (IFN) and IFN-inducible proteins changes after infection with SFTSV and transfection of NSs. Our recent studies have shown that NSs inhibit the activation of IFN promoters due to NS-dependent degradation of RIG-I via a ubiquitin-dependent pathway, thus blocking the activation of the downstream signaling pathways and inhibiting the production of IFN-β (unpublished data). Moreover, in infected and transfected cells, the NSs can form viroplasmlike structures (Wu et al. 2014) and affect virus replication (Lei et al. 2015). These observations suggest that NSs might play a significant role in viral infection.
Patients with SFTSV infections often develop acute fever, accompanied by thrombocytopenia, leukocyte reduction, gastrointestinal dysfunction, myaμgia, regional lymph node swelling, and other symptoms, and patients with severe infections can eventually progress to multiple organ failure or even death (Yu et al. 2011; Sun et al. 2012a; Liu et al. 2014). Although SFTSV poses a serious threat to public health, in spite of its recent isolation, the pathogenesis of this virus remains unclear. Notably, no effective vaccines or U.S. Food and Drug Administration (FDA)-approved antivirals have been introduced to date, which can be used to treat or prevent SFTS.
The proteasome system, as a major non-lysosomal protein degradation system in eukaryotic organisms, plays an important regulatory role in cell proliferation, differentiation, and protein degradation. In addition, it participates in the invasion of pathogens, pathogenicity, and immune response of the organisms. Studies have revealed that many viruses use proteasome systems to promote their replication and release. Therefore, inhibition of the proteasome degradation pathway is an effective way to inhibit virus proliferation. Bortezomib (PS-341) is an FDA-approved and highly selective reversible proteasome inhibitor with demonstrated antiviral activity (Choy et al. 2015; Keck et al. 2015). To explore whether PS-341 can be used as an effective SFTSV replication inhibitor, we analyzed the antiviral efficacy of PS-341 against SFTSV infection and revealed its killing effect prior to viral invasion of the susceptible cells. Further, its inhibitory effect on viral replication and release was determined. In addition, we elucidated the mechanism underlying SFTSV-encoded NS-mediated degradation of RIG-I. Our research may provide a basis for finding new anti-SFTSV drugs.
-
Bortezomib (PS-341; cat. no. HY-10227) was purchased from MedChem Express. Ac-YVAD-CHO (cat. no. ALX-260-027-M005) was purchased from Enzo Life Science. Staurosporine (STS; cat. no. 62996-74-1) was purchased from Active Biopharma Corp. MTT [3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; cat. no. M1020)] and DAPI (4', 6-diamidino-2-phenylindole; cat. no. H-1200) were acquired from Solarbio Life Science. The enhanced chemiluminescence (ECL) reagent was obtained from Thermo Fisher Scientific Inc. (USA). The primary antibodies against β-Tubulin (cat. no. AC021), anti-HA (cat. no. AE008) and anti-Flag (cat. no. AE005) were purchased from ABclonal technology. The primary antibodies against NP was obtained from Prof. Mifang Liang (China, CDC).
-
The SFTSV strain HB29 was obtained from Prof. Mifang Liang (China, CDC). The Sendai virus (SeV) strain BB1 was obtained from Prof. Lishu Zheng (China, CDC). All experiments were performed with human embryonic kidney HEK293T (293T) or Africa green monkey kidney epithelial cells (Vero) maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (100 IU/mL) and incubated in 5% CO2 at 37 ℃. When cells adhered to the bottom of the flask and attained approximately 80%–90% confluence, then PS-341 was added and the control group was treated with dimethyl sulfoxide (DMSO). A 1:400, 000 dilution of DMSO was prepared in phosphate buffered solution (PBS). PS-341 antiviral ability was analyzed according to published protocols, with modifications as described below (Li et al. 2015).
-
For cell viability analysis, 293T cells were cultured in a 96-well plate at 37 ℃ overnight. PS-341 was then added at the final concentrations of 0, 6.25, 12.5, 25, 50, 100, 200, 400, 600 or 800 nmol/L and further cultured for 24 h. The cell viability was determined by the MTT assay (cat. no. M1020; Solarbio Life Science) according to instructions.
-
Based on previous report (Li et al. 2015), four different assays were used to analyze the antiviral ability of PS-341, as described below.
Method 1: SFTSV was co-cultured with PS-341 at 37 ℃ for 4 h; Method 2: the virus and PS-341 were co-adsorbed for 1 h; Method 3: the cells were infected with the virus for 1 h before PS-341 was added; Method 4: the cells were pretreated with PS-341 for 2 h before adsorbing the virus, after the above treatment, the medium was aspirated, and the cells were washed three times with DMEM to remove any unabsorbed SFTSV and added fresh medium containing PS-341 (Fig. 1B).
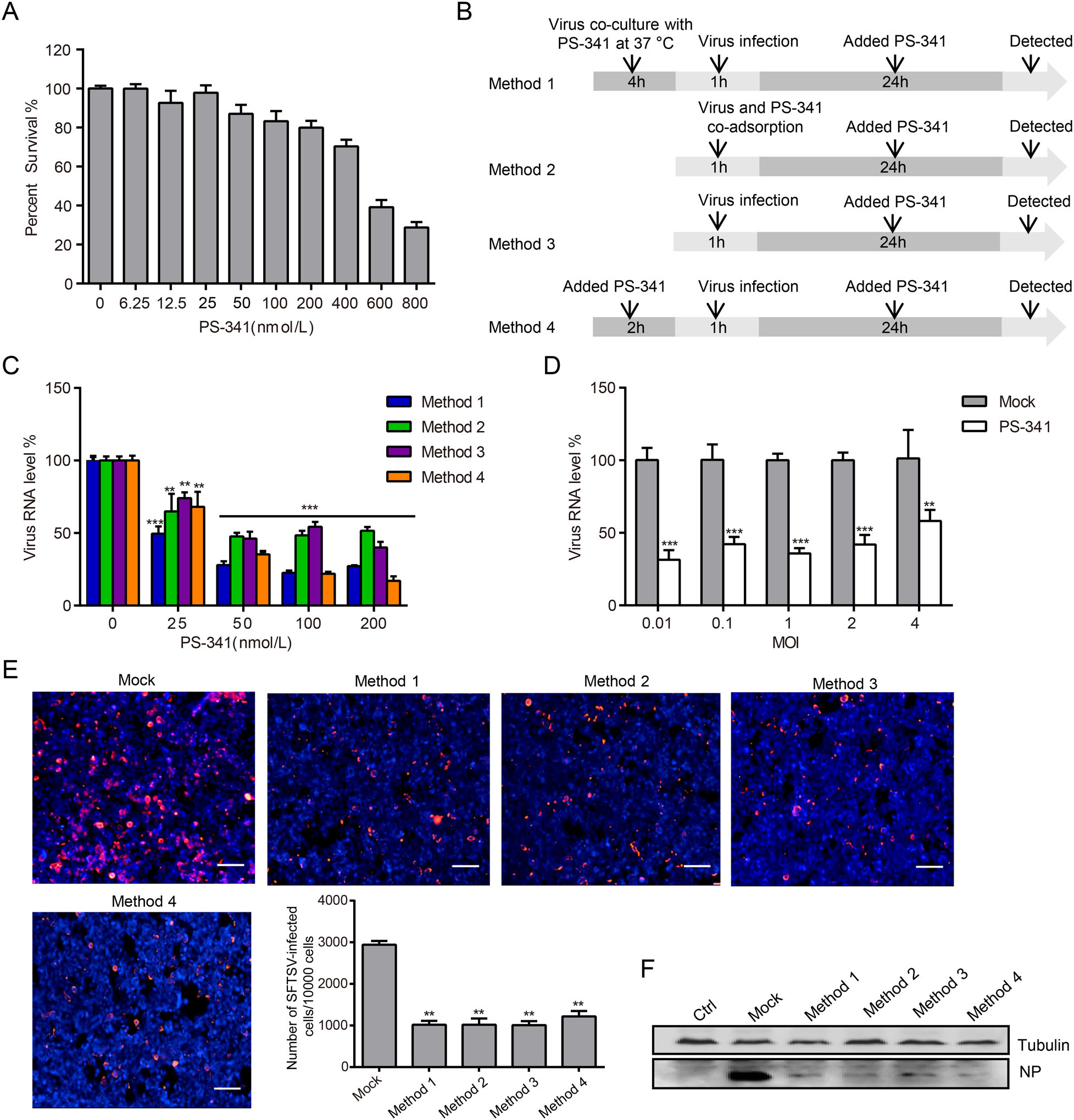
Figure 1. Toxicity and efficacy of PS-341 in 293T cells. A 293T cells were each treated with 0, 6.25, 12.5, 25, 50, 100, 200, 400, 600 or 800 nmol/L PS-341, and analyzed for survival using the MTT assay at 24 h. B 293T cells were treated with PS-341 as described in the schematic diagram. C With SFTSV (MOI=0.1) infection, 293T cells were each treated with 0, 25, 50, 100, or 200 nmol/L PS-341 for 24 h under four treatment conditions. The infected cells were collected, and measured by qRT-PCR. Asterisk indicates significant difference in viral RNA level between PS-341 untreated group and PS-341 treated group, **P < 0.01, ***P < 0.001. D 293T cells were incubated with SFTSV (MOI 0.01, 0.1, 1, 2 or 4) for 1 h then and cultured in medium containing DMSO (Mock) or PS-341 (50 nmol/L) for 24 h. The viral RNA in the whole cell lysate was extracted for qRT-PCR analysis. Asterisk indicates significant difference in viral RNA level between the mock group and PS-341 treated group at the same virus infected titer, **P < 0.01, ***P < 0.001. E 293T cells were infected with SFTSV (MOI = 1) and treated with PS-341 (50 nmol/L) under four treatment conditions for 24 h and detected by immunofluorescence with rabbit anti-NP primary antibody and followed by secondary antibody labeled with TRITC. DAPI was used to counterstain the nuclei. The histogram shows the statistical analysis of 293T cell fluorescence image (Bar = 30 μm). Asterisk indicates significant difference in viral RNA level between Mock and PS-341 -treated group, **P < 0.01. F The total protein of cells was extracted and detected by Western blotting. Ctrl: uninfected cells; Mock: SFTSV-infected cells. All samples were run in triplicate, and the experiment was repeated three times.
To detect the dose-dependent inhibitory effect of PS-341 on SFTSV, 293T cells were each pre-treated with 0, 25, 50, 100, or 200 nmol/L PS-341 with four assays. After adsorbing SFTSV (MOI 0.1) for 1 h, 293T cells were cultured in medium containing different concentrations of PS-341 for 24 h, and the viral RNA was then detected via quantitative reverse transcription PCR (qRT-PCR).
To evaluate the efficacy of PS-341 inhibit SFTSV replication at different multiplicity of infection (MOI), 293T cells were incubated with SFTSV (MOI 0.01, 0.1, 1, 2 or 4) for 1 h, then washed with DMEM to remove unabsorbed virus, and cultured in medium containing DMSO (Mock) or PS-341 (50 nmol/L). The cells were collected at 24 h, and the viral RNA in the whole cell lysate was extracted for qRT-PCR analysis.
To assess the killing effect of PS-341 prior to viral invasion of the cells, PS-341 (50 nmol/L) and DMSO (Mock) were incubated with SFTSV (MOI 0.1) at 37 ℃ for 0, 2, 4, or 8 h. SFTSV was absorbed for 1 h, the medium was aspirated, and fresh medium was then added. The infected cells and supernatants were collected at 24 h, and total RNA was then extracted for subsequent qRT-PCR analyses.
To investigate whether PS-341 inhibit the replication of SFTSV by inducing apoptosis, the 293T cells and Vero cells were incubated with SFTSV (MOI 0.1) for 1 h, and then cultured in medium containing PS-341 (50 nmol/L) & STS (200 nmol/L) or PS-341 (50 nmol/L) & Ac-YVADCHO (40 μmol/L). The cells were collected at 24 h for qRT-PCR analysis.
-
Total RNA was extracted using the QIAzol Lysis Reagent (cat. no. 79306) which was purchased from QIAGEN. RNAs were reverse transcribed to cDNA with the TransScript First-Strand cDNA Synthesis SuperMix (cat. no. AT301; Transgen Biotech). S-segment-specific primers (Sun et al. 2012a, b) were used to determine the levels of viral gene mRNAs, and the levels of viral mRNAs were normalized to GAPDH mRNA.
-
PS-341 treated 293T cells or Vero cells were fixed with paraformaldehyde (4%) for 15 min, permeabilized with 0.5% Triton-100 for 10 min, blocked with 1% bovine serum albumin for 20 min, and then probed with rabbit anti-NP primary antibody and followed by secondary antibody labeled with TRITC. DAPI was used to counterstain the nuclei. Mass data acquisition and statistical analysis were performed using LionheartTM FX Automated Microscope and Gen5 Software BioTek Instruments Inc. (Winooski, VT, USA).
-
Cells were homogenized with lysis buffer, the samples were separated on 10% SDS-PAGE and transferred onto a nitrocellulose membranes (cat. no. 66485; Pall Corporation). After blocking with 5% nonfat milk for 1 h, the membranes were probed with primary antibodies (rabbit anti-NP, mouse anti-Tubulin, mouse anti-HA or mouse anti-Flag) and followed by secondary antibody labeled with HRP. Subsequently, enhanced chemiluminescence was used to detect the immunoreactive bands, and images of the blots were recorded using the ChemiDocTM XRS+ gel imaging system (Bio-Rad, USA).
-
The TransLipid HL transfection reagent (cat. no. FT111; Transgen Biotech) was used to transfect 293T cells. After 48 h, the cells were collected and treated. According to the TransDetect Double-Luciferase Reporter Assay Kit (cat. no. FR201; Transgen Biotech) standard operation steps, the luciferase signals was detected by the Centro XS3 LB 960 microplate luminometer (Berthold Technologies, Germany).
-
All results were analyzed with GraphPad Prism and presented as mean ± SD. Statistical significance was determined using two-tailed Student's unpaired t test (*P < 0.05, **P < 0.01, ***P < 0.001).
Reagents and Chemicals
Viruses and Cell Lines
Cytotoxicity Assay of PS-341
Cell Culture and Viral Infection
Quantitative Real-Time PCR for Viral RNA
Immunofluorescence
Western Blotting
Dual-Luciferase Reporter System
Statistical Analysis
-
To elucidate the effects of PS-341 against SFTSV infections, we first determined its toxicity to 293T cells, a cell line that serves as a model for studying SFTSV infections. The results showed that the survival rates of the 293T cells in the PS-341 (50 nmol/L-treated) group were 90% at 24 h, compared with the PS-341 untreated group (Fig. 1A). The 50% cytotoxic concentration (CC50) of PS-341 in 293T cells was above 500 nmol/L at 24 h. Further, by using medium containing different concentrations of PS-341 to culture virus-infected cells, we found that PS-341 demonstrated a dose-dependent inhibition of SFTSV replication (Fig. 1B, 1C). Based on these results, we used a 50 nmol/L concentration of PS-341 in subsequent experiments. The inhibitory effects of PS-341 on SFTSV replication at different MOI (0.01, 0.1, 1, 2 or 4) was also evaluated (Fig. 1D).
The data presented thus far suggested that PS-341 could effectively inhibit SFTSV replication at the mRNA level. To further analyze the effect of PS-341 on the replication of SFTSV in cells, we used immunofluorescence and immunoblotting analyses to characterize the content of the virus proteins in the cells. The 293T cells were each treated with 50 nmol/L PS-341 for 24 h under the four methods and then fixed and incubated with anti-NP antibodies for immunofluorescence analysis (Fig. 1E). The images were assessed using high content analysis. For every 10, 000 cells, the infection rate of SFTSV was measured to be approximately 30% in the 293T cells (Fig. 1E, Mock lane). However, compared with the Mock group, when the 293T cells were treated with 50 nmol/L PS-341 under the four treatment conditions, the number of cells infected by SFTSV demonstrated an approximately 60% reduction (Fig. 1E). After SFTSV (MOI = 1) infection for 24 h, the expression of the NP protein was then assessed in 293T cells via immunoblotting. Similarly, we found that PS-341 could effectively inhibit the expression of NP (Fig. 1F). The same phenomenon of inhibiting virus replication was also observed in Vero cells (Supplementary Figure S1). These data further illustrated that PS-341 could effectively inhibit the proliferation of SFTSV.
-
Antiviral drugs are used to limit or eradicate viral infections by directly inhibiting or killing viruses, interfering with virus adsorption, preventing viruses from invading cells, inhibiting viral biosynthesis and release, and/or enhancing the antiviral response of the host. In order to assess the killing effect of PS-341 prior to the virus invasion of the cells, PS-341 (50 nmol/L) and DMSO (Mock) were incubated with SFTSV (MOI = 0.1) at 37 ℃ for 0, 2, 4, or 8 h. SFTSV was absorbed for 1 h, the medium was aspirated, and fresh medium was then added. The infected cells and supernatants were collected at 24 h, and total RNA was then extracted for subsequent qRT-PCR analyses. We found that the RNA levels of SFTSV in infected cells and supernatants significantly decreased after being incubated with PS-341 for 4 h and 8 h at 37 ℃, compared with the DMSO group at the same incubation time point (Fig. 2A, 2B), indicating that PS- 341 could directly inhibit the infectivity of the SFTSV.
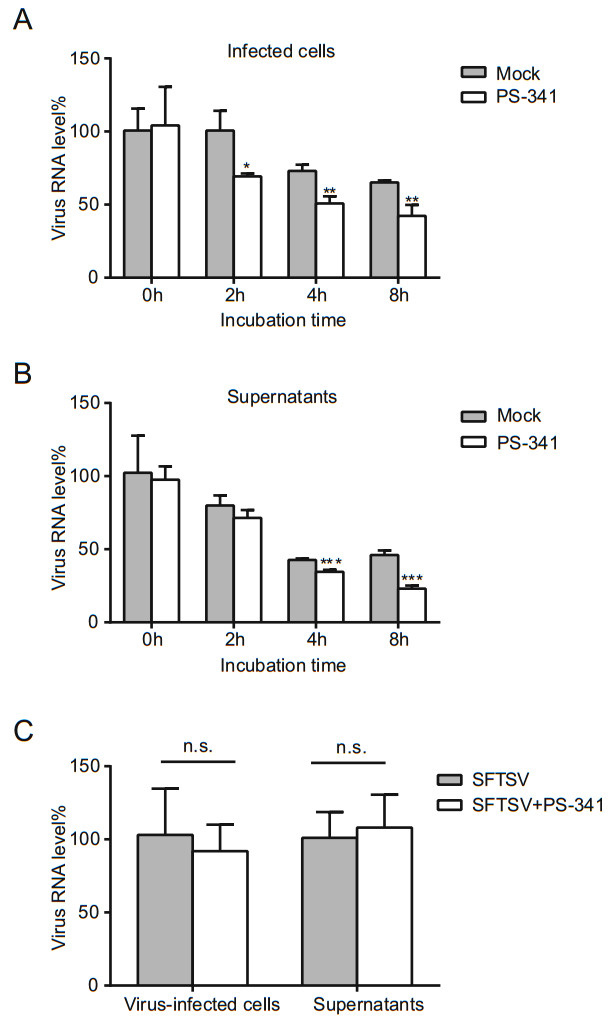
Figure 2. The effect of PS-341 on SFTSV infectivity and absorption. SFTSV (MOI = 0.1) was pre-treated with 50 nmol/L PS-341 or DMSO (Mock) for 0, 2, 4, or 8 h p.i. at 37 ℃, and 293T cells were infected with the mixture for 1 h., After washed three times with DMEM, fresh medium were added. The cells (A) and supernatant (B) were collected at 24 h for qRT-PCR analysis. Asterisk indicates significant difference in viral RNA level between mock and PS-341 treated group at the same incubation time point, *P < 0.05, **P < 0.01, ***P < 0.001. C 293T cells were co-cultured with 50 nmol/L PS-341 and SFTSV (MOI = 0.1) or with only SFTSV for 30 min at 4 ℃. The cells and supernatants were collected for qRTPCR detection. n.s., P> 0.05. All samples were run in triplicate, and the experiment was repeated three times.
As the virus enters the cell through the cell membrane, it is first adsorbed to the cell membrane in combination with a receptor; then, the ingestion of the virus is accomplished (to a certain extent) by the endocytosis of active extracellular macromolecules. 293T cells were co-cultured with 50 nmol/L PS-341 and SFTSV (MOI = 0.1) or with only SFTSV for 30 min at 4 ℃. Then the cells and supernatants were collected immediately and subjected to qRT-PCR analysis. No notable differences were observed in the inhibition rates of PS-341 against SFTSV with regard to the virus absorption assays in 293T cells (Fig. 2C). These results indicated that PS-341 exhibited antiviral activity due to direct anti-virus or prophylactic activities, but inhibition of viral absorption was not observed.
-
In our above studies, we have shown that PS-341 can inhibit the infectivity of SFTSV directly. We further investigated whether PS-341 could inhibit SFTSV replication in 293T cells. 293T cells were treated under the four treatment conditions as described before. After 1 h of virus adsorption, the supernatant was discarded and the cells were washed three times with DMEM. Then the fresh medium containing PS-341 (50 nmol/L) was added. The cells were collected at different time points for qRT-PCR analysis and the virus proliferation curves were drawn. The results showed that PS-341 significantly inhibited SFTSV replication in four pretreatment methods in the 293T cells (Fig. 3A). In addition, we also detected the virus titer in the supernatant via qRT-PCR, and the results indicated that virus release was also inhibited in 293T cells (Fig. 3B). To analyze the antiviral effect of PS-341 at different points in the viral replication cycle of SFTSV, after virus absorption for 1 h, PS-341 (50 nmol/L) was added to 293T cells at 4 h, 8 h, 12 h, and 24 h post-infection (p.i.), respectively. The infected cells (Fig. 3C) and supernatants (Fig. 3D) were collected at 48 h p.i., and time-course analysis showed that PS-341 could suppress SFTSV replication and release in 293T cells; the same test was also performed in Vero cells (Supplementary Figure S2).
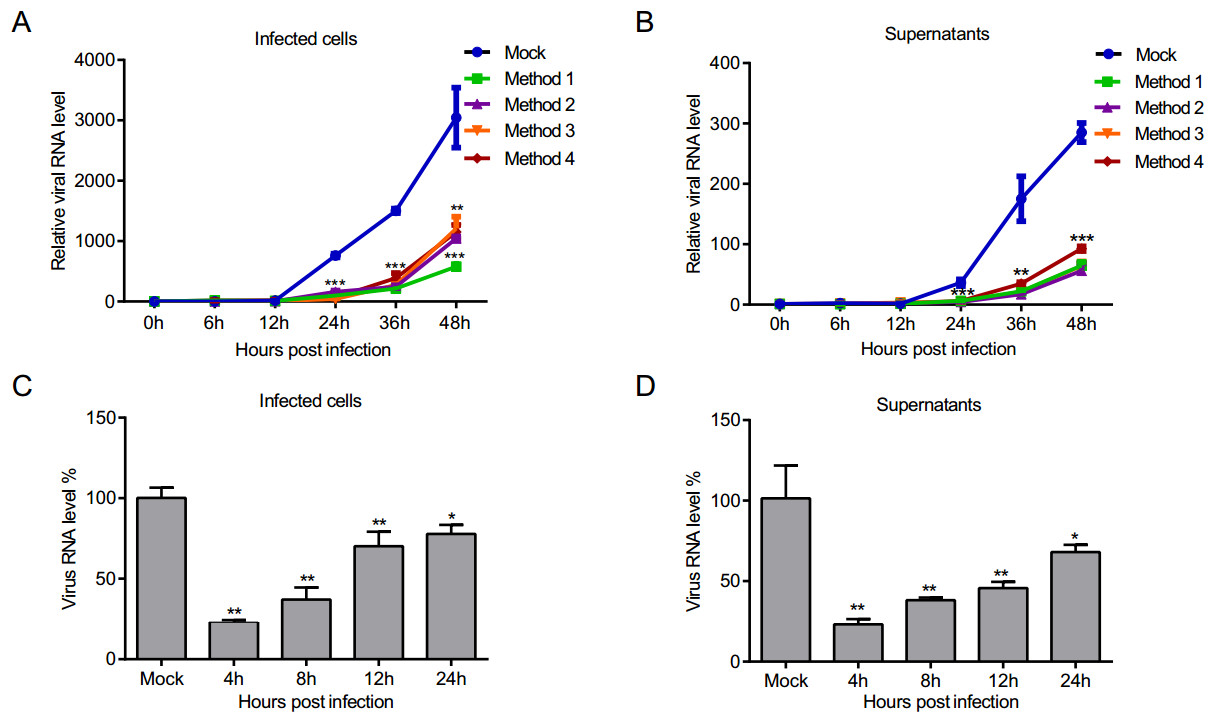
Figure 3. The inhibitory effect of PS-341 on SFTSV replication and release. 293T cells were PS-341 (50 nmol/L)-treated under four conditions, the infected cells (A) and culture supernatants (B) were collected at particular points of time, and analyzed by qRT-PCR. The relative viral RNA level was expressed as fold change relative to the value of viral RNA at 0 h (set as 1). Asterisk indicates significant difference in viral RNA level between Mock and PS-341-treated group, **P < 0.01, ***P < 0.001. C, D After SFTSV absorption for 1 h, PS-341 (50 nmol/L) was added to 293T cells at 4, 8, 12, and 24 h p.i. The infected cells (C) and supernatants (D) were collected at 48 h p.i. for qRT-PCR detection. Asterisk indicates significant difference in viral RNA level between Mock and PS-341-treated group. *P < 0.05, **P < 0.01, ***P < 0.001. All samples were run in triplicate, and the experiment was repeated three times.
-
Bunyaviruses cause significant human morbidity and mortality (Xu et al. 2011; Yu et al. 2011; Zhang et al. 2011). In reaction to the host innate immune response, phleboviruses target specific cellular proteins and can strategically block the effects of type Ⅰ IFNs. Studies have revealed that phlebovirus infection inhibits the synthesis of type Ⅰ IFNs, and the viral NSs were identified as an IFN antagonist (Qu et al. 2012; Ning et al. 2014). Previous studies have also shown that PS-341 treatment promotes IFN expression through the NF-κB and JNK/AP-1 pathways, leading to suppression of virus multiplication (Dudek et al. 2010). We first examined the RNA level of IFN-β in Sendai virus (SeV)-infected cells after DMSO or PS-341 treatment. The results revealed that SFTSV inhibited the SeV-activated IFN-β signaling pathway, but IFN-β RNA level was recovered in PS-341-treated cells (Fig. 4A).
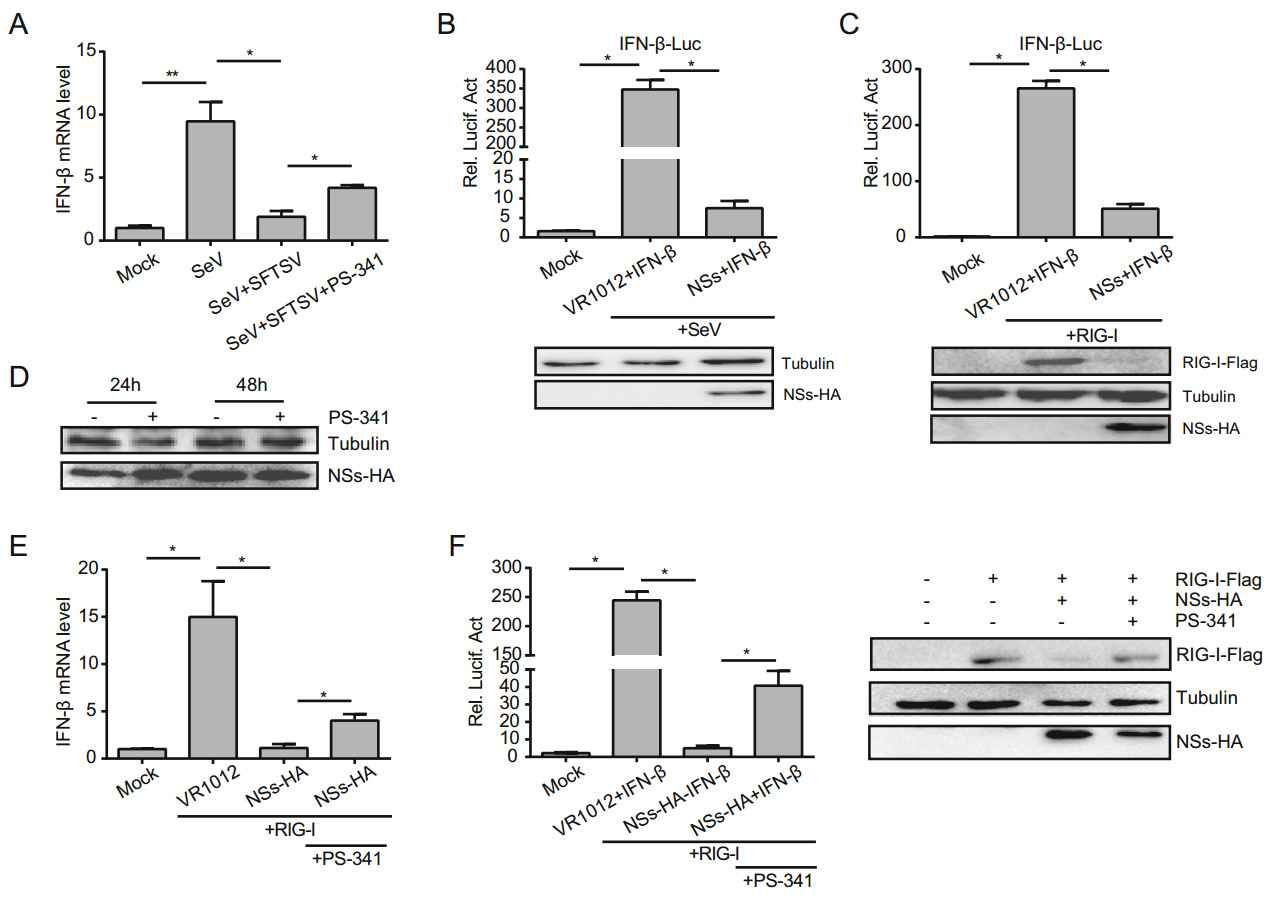
Figure 4. PS-341 suppresses the NSs-mediated degradation of RIG-I. A After infected with SFTSV (MOI = 1), 293T cells were infected with SeV (20 HA/mL) and treated with DMSO or PS-341 (50 nmol/ L). Then the cells were collected at 24 h, the mRNA levels of IFN-β were analyzed by qRT-PCR. The relative IFN-β RNA level was expressed as fold change relative to the value of IFN-β RNA in mock group (set as 1). B 293T cells (5 × 105) were co-transfected with the IFN-β luciferase reporter plasmid (200 ng), pRL-SV40 plasmid (1 ng), and NSs-HA plasmid (800 ng) or VR1012 plasmid (800 ng). After 24 h, cells were infected and incubated with SeV (20 HA/mL) for another 16 h, and then luciferase activities were detected. C 293T cells (5 × 105) were transfected with the same IFN-β luciferase reporter plasmid (200 ng), pRL-SV40 plasmid (1 ng), RIG-I-Flag plasmid (400 ng), NSs-HA plasmid (400 ng) or VR1012 plasmid. After a 48 h culture period, luciferase activities were detected. D 293T cells (5 × 105) were transfected with NSs-HA plasmid (500 ng) and then treated with DMSO or PS-341 for 24 or 48 h and detected the expression of NSs-HA by Western blotting. E, F 293T cells were also transfected with the same plasmids as (C), and after 24 h, PS-341 was added to cells before qRT-PCR test (E) or detected the luciferase activities (F). The relative IFN-β RNA level was expressed as fold change relative to the value of IFN-β RNA in Mock group (set as 1). The expression level of proteins was determined by immunoblotting analysis. All samples were run in triplicate, and the experiment was repeated three times. *P < 0.05, **P < 0.01.
As known, NSs play an important role in the replication of SFTSV and in the regulation of the host response. To further investigate the relationship between NSs and IFN-β, 293T cells were co-transfected with the NSs-HA plasmid, the IFN-β luciferase reporter plasmid and the pRL-SV40 plasmid. The vector plasmid (VR1012) was used as control. At 24 h, we added SeV to activate the IFN signaling pathway. The effect of NSs on the activation of the IFN-β promoter stimulated by SeV infection was analyzed via dual-luciferase reporter assay, and we found that the NSs could inhibit the activation of the IFN-β promoter (Fig. 4B).
Gori-Savellini et al. (2013) have revealed that the Tuscana virus (TOSV) NSs degrade RIG-I via an ubiquitindependent pathway, blocking the activation of downstream signaling pathways and inhibiting the production of IFN-β. The RIG-I-Flag plasmid was transfected into 293T cells to activate the IFN promoter. Dual-luciferase reporter assay indicated that the NSs of the SFTSV significantly inhibited the activation of the RIG-I–activated IFN-β promoter (Fig. 4C). Moreover, Western blot analysis showed that PS-341 did not affect the expression of NSs in these cells (Fig. 4D). We also found that NSs induced the degradation of RIG-I, as based on Western blotting (Fig. 4C). Additionally, qRT-PCR (Fig. 4E) and dual-luciferase reporter assay (Fig. 4F) indicated that PS-341 could inhibit the NS-mediated degradation of RIG-I, thereby antagonizing the inhibitory effect of NSs on IFN.
-
Apoptosis and innate immunity are closely related to each other and are physiological processes strictly regulated by the body. These pathways play important roles in the process of disease development, including virus clearance, innate immune activation, and identification and presentation of viral antigens. PS-341 can promote the activation of the proapoptotic c-Jun-NH2 terminal kinase and inhibit the degradation of p21, p27, and p53 (Davies et al. 2005). Previous studies have shown that, under the four different treatment conditions, the inhibition rate of PS-341 on SFTSV was 50%~70% in 293T cells, only ~40% of Vero cells would be inhibited at 24 h (Fig. 5A). We also assessed the virus titer in the supernatant by qRT-PCR, and the results indicated that ~90% of virus release was inhibited in 293T cells, but only ~25% was inhibited in Vero cells at 24 h (Fig. 5B). However, the gene for IFN synthesis is defective or absent in Vero cells (Emeny and Morgan 1979). Therefore, other pathways must exist that allow PS-341 to inhibit the replication and release of SFTSV. Previous data has shown that the cell survival rates decreased at 24 h when using PS-341 at concentrations over 50 nmol/L; thus, we suspected that PS-341 could inhibit SFTSV replication by inducing apoptosis. Subsequently, we used staurosporine as an inhibitor of nonselective protein kinase C (PKC), which inhibits PKC and tyrosine kinase and is widely used to induce apoptosis in most cell models (Tamaoki et al. 1986; Ruegg and Burgess 1989; Yuste et al. 2005). Staurosporine preferentially activates caspase-dependent apoptosis. We used staurosporine (STS, 200 nmol/L) or Ac-YVAD-CHO (40 μmol/L), a reversible caspase-1, 4 inhibitor, with PS-341 co-processed cells and analyzed the SFTSV mRNA level via qRT-PCR. We found that when treated with STS and PS-341, the inhibition of SFTSV was stronger. When treated with Ac-YVAD-CHO and PS-341, Ac-YVADCHO reversed the inhibitory effect of PS-341 on SFTSV in both 293T and Vero cells (Fig. 5C, 5D).
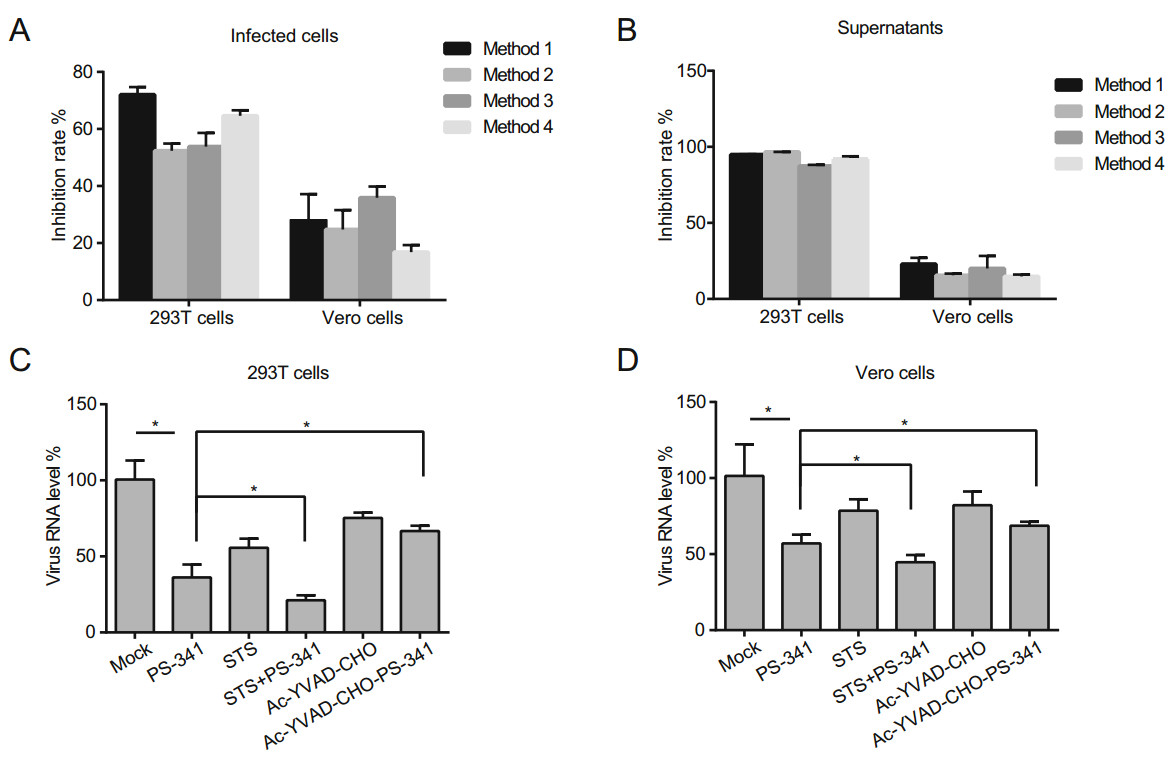
Figure 5. PS-341 inhibits the replication of SFTSV by inducing apoptosis. A, B 293T and Vero cells were each treated with 50 nmol/L PS-341 under four conditions, the infected cells (A) and supernatants (B) were collected at 24 h, and analyzed by qRT-PCR. The virus RNA level in PS-341 untreated group was set as 100%, the inhibition rate of PS-341 to SFTSV was calculated and plotted under four conditions. C 293T cells and D Vero cells were infected with SFTSV (MOI = 0.1) and treated with PS-341 (50 nmol/L) and STS (200 nmol/L) or PS-341 (50 nmol/L) and Ac-YVAD-CHO (40 μmol/L) for 24 h, and then collected for qRT-PCR analysis. All samples were run in triplicate, and the experiment was repeated three times. *P < 0.05.
PS-341 Treatment Decreases SFTSV Proliferation in Susceptible Cells
The Effect of PS-341 on SFTSV Infectivity and Absorption
PS-341 Suppresses SFTSV Replication and Release
PS-341 Inhibits NS-Mediated Degradation of RIG-I
PS-341 Inhibits the Replication of SFTSV by Inducing Apoptosis
-
SFTS is a serious disease with a high mortality rate that has spread to several parts of the world (Yu et al. 2011; Chang and Woo 2013; Li et al. 2014; Park et al. 2014). Antiviral drugs that can be used against SFTSV are limited, so we urgently need new compounds to combat this disease. However, working towards a new drug through the basic research process for approval is very time-consuming, and the process may easily exceed 10 years or more. An alternative and more rapid development strategy for antiviral drugs is to reuse the existing licensed drugs (Tricou et al. 2010; Nguyen et al. 2013; Low et al. 2014).
The severity of SFTSV infection and viral replication are governed by the host immune responses. Because RNA viruses require cellular mechanisms to replicate, we hypothesized that inhibition of vital cell targets might be a suitable approach for inhibiting viral replication, and such an approach may also help to overcome problems associated with drug resistance (Ludwig 2009; Lee and Yen 2012; Planz 2013; Watanabe and Kawaoka 2015). The proteasomal degradation pathway is a vital mechanism that regulates many cellular processes (Wang and Maldonado 2006), including the activation and inhibition of transcriptional targets, signal transduction, apoptosis, and the cell cycle. Some viruses are capable of targeting cellular proteins for proteasome-specific degradation. Notably, proteasome-specific degradation is an important step in the replication of several viruses (Thomas et al. 1999; Klinger and Schubert 2005). Therefore, drugs that inhibit the function of the proteasomes are effective for inhibiting the progression of the viral life cycle. In this study, PS-341, an inhibitor of the 26S proteasome, was shown to suppress SFTSV infectivity, replication, and release. PS-341 is an effective and selective FDA-approved proteasome inhibitor (Adams and Kauffman 2004) that has demonstrated in vitro activity against viruses, including Rift Valley fever virus (RVFV) (Keck et al. 2015) and Dengue virus (Choy et al. 2015). In contrast to the results obtained for RVFV, in which PS-341 inhibited RVFV infection by abolishing the ability of NSs to form nuclear filaments (Keck et al. 2015), PS-341 apparently employs distinct mechanisms to target SFTSV.
The type Ⅰ IFN system is an important part of the innate immune system and facilitates adaptive immunity to inhibit viral infections. Thus, to counteract IFN signaling, many viruses have evolved multiple strategies to overcome this defense by targeting specific signaling proteins, suppressing their phosphorylation and activation or inducing their degradation. The NSs of the La Crosse virus (family, Bunyavirus) have been demonstrated to be effective inhibitors of transcription and antagonists of the IFN signaling pathway in host cells (Blakqori et al. 2007). SFTSV NSs form inclusion body (IBs) in the cytoplasm, acting as collection sites for multiple components of the signaling cascade and serves to block IFN production (Santiago et al. 2014; Wu et al. 2014; Ning et al. 2015). Previous studies have shown that NSs are isolated from the TBK1/IKKε/ IRF3 complex and localized to the viral cytoplasmic VLSs, effectively 'hijacking' RIG-I and TRIM25 (an E3 ligase) to the VLSs, isolating the signaling molecule IRF3, and blocking the activation of the downstream signal pathway (Ning et al. 2014; Wu et al. 2014). SFTSV NSs can antagonize the production of IFN-β by inducing RIG-I degradation (Fig. 4C). After addition of PS-341, the expression of RIG-I was restored, and the activity of the IFN-β promoter was also recovered (Fig. 4F). However, in Vero cells, IFN synthesis is defective or absent; however, after PS-341 treatment, SFTSV infection was still blocked. This indicated that PS-341 could inhibit the proliferation of SFTSV by a mechanism independent of the IFN system.
PS-341 can inhibit NF-κB activity by inhibiting the ubiquitin-proteasome system (UPS) (Altun et al. 2005), and it also inhibits the degradation of p53 by the 26S proteasome (Marine and Lozano 2010), thus inhibiting cell adhesion and cell proliferation as well as promoting cell apoptosis. However, this was dependent on the concentration of PS-341 used, the duration of action, and the type of cell. After treatment of the cells with the apoptosisinducing agent STS, the replication of SFTSV was inhibited, indicating that apoptosis inhibited the proliferation of this virus. When treated with Ac-YVAD-CHO and PS-341, Ac-YVAD-CHO reversed the inhibitory effect of PS-341 on SFTSV (Fig. 5C, 5D). This indicated that PS-341 could also affect the replication of SFTSV by inducing apoptosis. The specific mechanism underlying this observation is not well-understood and needs to be studied in further detail. It is clear that PS-341 can inhibit the replication of SFTSV by interfering with multiple pathways, included the interferon and apoptosis pathways; however, the correlation between these two pathways and their primary and secondary effects are unclear. Moreover, it is likely that different concentrations of PS-341 can induce different mechanisms.
Taken together, these data demonstrate that PS-341 blocked SFTSV infection by affecting virus infectivity, replication, and release. Additionally, we also revealed that PS-341 inhibits SFTSV proliferation by affecting the IFN system and host cell apoptosis. To the best of our knowledge, this study is the first to define PS-341 as a promising antiviral agent against SFTSV. With its clinical application, PS-341 could serve as a feasible and safe short-term treatment for acute SFTSV infections.
-
We thank Mifang Liang (Chinese Center for Disease Control and Prevention) for providing critical reagents, and Minglei Pan (School of Life Sciences, Tianjin University) for advice and technical assistance. This work was supported by the National Science Foundation of China (31270201), the National Key Research and Development Program of China (2017YFA0205102), the Seed Foundation of Tianjin University (2014XRX-0026), and the National Science Foundation of Tianjin (No.16JCQNJC09800).
-
TW and ZW conceived and designed the experiments. SL and HL performed the experiments and analyzed the data. KZ, XL, and YD contributed reagents/materials/analysis tools. SL wrote the manuscript and prepared the figures. TW and ZW revised the manuscript, organized the collaboration, and directed the project. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
















 DownLoad:
DownLoad: