HTML
-
Rice is one of the most important food crops worldwide and more than 90% of rice grains are produced in Asia (Bheemanahalli et al. 2016). Many plant viruses are known to cause devastating diseases to rice productions each year. Among these viruses, Rice stripe mosaic virus (RSMV) is first discovered in rice fields in the southern part of China in 2015 (Yang et al. 2017a), and is the only virus belonging to the genus Cytorhabdovirus in family Rhabdoviridae. RSMV is transmitted by leafhopper Recilia dorsalis in a persistent and propagative manner (Yang et al. 2017a). Rice strip mosaic disease caused by RSMV often shows slight plant dwarfing, yellow streaks or mosaic in leaves, twisted leaf tips, increase of tiller number, and empty rice panicles (Yang et al. 2017a, b, 2018).
RSMV virion is bacilliform with 300–375 nm in length and 45–55 nm width, and contains a negative-sense RNA genome (Yang et al. 2017a). Similar to most Rhabdoviruses, RSMV complementary RNA contains seven open reading frames (ORFs), encoding two non-structural proteins (P3 and P6) and five structural proteins (nucleocapsid protein, glycoprotein, ribonucleic acid polymerase, phosphorylated protein and matrix protein) (Jackson et al. 2005; Ammar et al. 2009; Kormelink et al. 2011; Afonso et al. 2016; Dietzgen et al. 2017; Yang et al. 2017a).
To study the epidemiology of RSMV and to develop a control strategy for this virus, people need specific and high throughput RSMV detection techniques. Current RSMV detections rely on biological symptom observation, Transmission electron microscopy (TEM), and reverse transcription polymerase chain reaction (RT-PCR) (Yang et al. 2017b). The biological symptom observation is conveniently used in fields. However, symptoms caused by different viruses are often indistinguishable. Both TEM and RT-PCR need expensive equipments and specialized working experiences. In contrast, serological methods are relatively simple, and specific and sensitive. Consequently, many reports consider that serological methods are most suitable for large-scale field survey (Chen et al. 2017; Zhang et al. 2018; Huang et al. 2019). In this study, we produced four highly sensitive and specific murine monoclonal antibodies (MAbs) against RSMV. We then developed an antigen-coated plate enzyme-linked immunosorbent assay (ACP-ELISA), a Dot-ELISA, and a Tissue print-ELISA for detections of RSMV in rice plants and leafhoppers. Analyses using field-collected rice and leafhopper samples showed that these three serological methods can be used to detect RSMV in infected samples quickly and accurately. We consider that these four MAbs and three serological methods are very useful for epidemiological studies and development of control strategies for RSMV.
-
Rice plants showing RSMV-like symptoms were collected from a paddy field in the Guangdong Province, China, and were individually tested for RSMV infection through RTPCR followed by gene cloning and DNA sequencing. Rice gall dwarf virus (RGDV), Rice stripe virus (RSV), Rice black-streaked dwarf virus (RBSDV) and Southern rice black-streaked dwarf virus (SRBSDV) were obtained previously (Wu et al. 2014) and maintained individually in authors' greenhouse. Leafhoppers Recilia dorsalis were fed on RSMV-infected rice plants to acquire RSMV and then used as viruliferous insects for the study. RSMV virions were purified from fresh RSMV-infected rice plant tissues as described previously (Yang et al. 2017a). Quality of the purified RSMV preparation was examined under an electron microscope (JEM -1200 EX, JEOL Ltd., Tokyo, Japan).
A total of 33 rice plants and 16 leafhoppers were collected from rice fields in the Guangdong Province of China during the 2018 rice-growing season, and tested for the presence of RSMV with the serological methods developed in this study.
-
The purified RSMV virions were used as an immunogen to immunize five eight-week-old BALB/c female mice through intraperitoneal injection as described previously (Song et al. 2017; Huang et al. 2019). Hybridoma cells secreting anti-RSMV MAbs, and ascitic fluids containing MAbs were prepared as described previously by (Chen et al. 2017). Titers of the resulting ascitic fluids were determined by an indirect enzyme-linked immunosorbent assay (indirect-ELISA) using purified RSMV virions (1 μg/mL) as the coating antigen. Isotypes of the four MAbs (1D4, 4A8, 8E4 and 11F11) were discriminated using a mouse MAb isotyping kit as instructed (Sigma-Aldrich, St. Louis, MO, USA). Specificity and sensitivity of the four MAbs were determined by Western blot assay and an antigencoated plate (ACP)-ELISA described previously (Shang et al. 2011; Wu et al. 2014).
-
Crude extracts from 33 field-collected rice plant tissues or homogenates from 16 leafhoppers were tested for RSMV infection using an ACP-ELISA described before (Wu et al. 2014; Liu et al. 2017). Crude extracts from a RSMV-infected and a healthy rice plant were used as the positive and negative controls, respectively. Similarly, homogenates from RSMV viruliferous and non-viruliferous leafhoppers were used as the positive and negative controls, respectively. Samples that had ACP-ELISA absorbance three times greater than that of the negative control were considered as RSMV-positive.
-
Dot-ELISA was performed as described (Wu et al. 2009). Briefly, rice plant tissues were ground in liquid nitrogen with mortars and pestles, and homogenized in 0.01 mol/L PBS (1 g tissue in 20 mL PBS). Leafhoppers were placed individually in 0.5 mL centrifuge tubes, containing 50 μL PBS each, and crashed with toothpicks. RSMV viruliferous and non-viruliferous leafhoppers were used as the positive and negative controls, respectively. Crude extracts of rice plants and leafhoppers were spotted individually onto nitrocellulose membranes (Amersham Biosciences, Bucks, UK; 2.5 μL per spot). After 10 min air dry at room temperature (RT), the membranes were blocked for 30 min in PBS solution containing 0.05% Tween 20 and 5% dried skimmed milk powder (PBST?M). The membranes were then incubated for 60 min in a diluted anti-RSMV MAb solution. After three rinses with PBST, the membranes were incubated for 60 min in a 1:8000 diluted alkaline phosphatase (AP)-or horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG secondary antibody solution. After four rinses with PBST, the membranes were incubated in a nitro blue tetrazolium (NBT)/5-bromo-4-chloro- 3-indolyl phosphate (BCIP) substrate solution or in a 3, 3', 5, 5'-tetramethylbenzidine (TMB) substrate solution (Promega, Madison, WI, USA) for color development. Samples developed a purple or a blue color within 10–20 min were considered to be RSMV-positive.
Tissue print-ELISA was performed as described previously (Liu et al. 2017). Briefly, rice stems were cut with a blade and the cut surface was quickly pressed against a nitrocellulose membrane for 3–5 s. Prints made with healthy or RSMV-infected rice stems were used as the negative and positive controls, respectively. Steps of antibody probing were the same as that described for Dot-ELISA.
-
Western blot was done as described (Wu et al. 2014). Tissues from RSMV-infected or healthy rice plants, or from RSMV viruliferous or non-viruliferous leafhoppers were tested using RSMV MAbs.
-
Total RNA was extracted from individual rice plant tissues or from leafhoppers using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). RSMV forward primer P3-F (5'- ATGAAGATCATCTGCAGTACTG-3', RSMV nucleotide position 3006–3027) and reverse primer P3-R (5'- TCAAGTAGCAAACTTGACATGG-3', RSMV nucleotide position 3518–3539) were designed using the conserved region in the P3 gene (GenBank accession MH720475.1). RT-PCR was performed using the SuperScriptTM Ⅲ One-Step RT-PCR System (Invitrogen, Carlsbad, CA, USA) and the P3-F/P3-R primer set. The resulting RT-PCR products were visualized in agarose gels, cloned, sequenced and compared with the known RSMV P3 gene sequences.
Virus Sources and Field Sample Collections
RSMV Monoclonal Antibody (MAb) Preparation
ACP-ELISA
Dot-ELISA and Tissue Print-ELISA
Western Blot
RT-PCR and DNA Sequencing
-
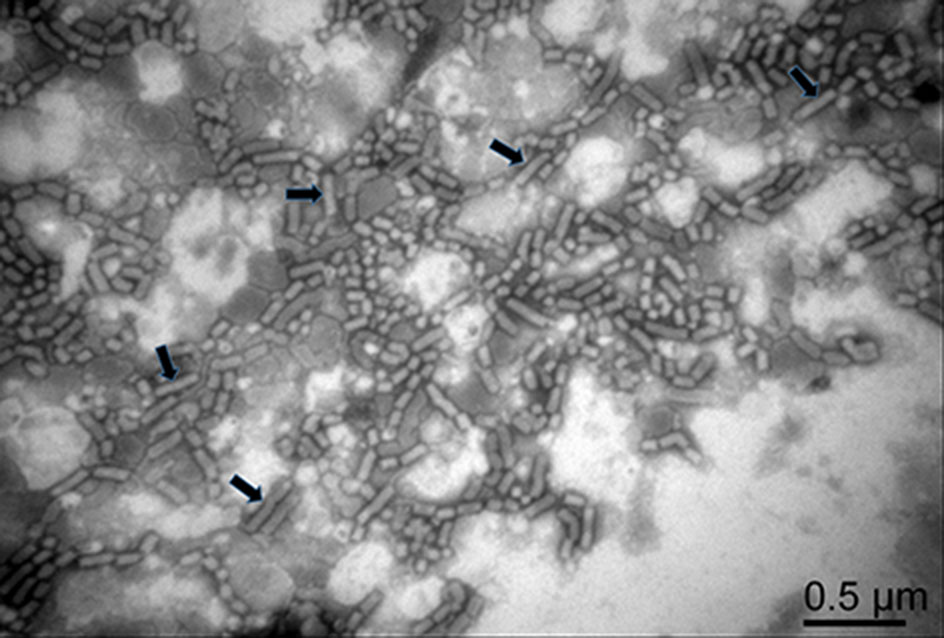
RSMV virions were purified from RSMV-infected rice plant tissues through differential centrifugation. Numerous bacilliform virions of 300–375 nm long and 45–55 nm wide, similar to viruses in the genus Cytorhabdovirus, were found in the purified preparation under a transmission electron microscope (Fig. 1, with black arrows). Virions shorter than 300–375 nm long were also observed in the preparation (Fig. 1), indicating that many virions were broken in the purified process.
-
Purified RSMV virions were used to immunize BALB/c mice through intraperitoneal injection. After four immunizations, spleen cells were isolated from immunized mice and used for hybridoma preparations. Four hybridoma cell lines secreting RSMV MAb (1D4, 4A8, 8E4 and 11F11) were generated through cell fusion, cell culture, antibody detection and then cell cloning. These four hybridoma cell lines were individually injected intraperitoneally into pristine-primed mice to produce ascites fluids. Yields of IgG in the four ascites fluids were found to range from 6.93 to 8.16 mg mL-1 (Table 1). Immunoglobulin class and subclass isoform of the four MAbs were identified as IgG1, kappa light chain (Table 1). Titers of the four MAbs in ascites were determined to be 10-7 by an indirect ELISA (Table 1).
MAb Isotype titer IgG yield (mg mL-1) 1D4 IgG1, κ chain 10-7 7.34 8E4 IgG1, κ chain 10-7 6.93 4A8 IgG1, κ chain 10-7 8.16 11F11 IgG1, κ chain 10-7 7.82 Table 1. Properties of the four anti-RSMV monoclonal antibodies.
Specificities of the four anti-RSMV MAbs were then determined by ACP-ELISA and Western blot, respectively. ACP-ELISA results indicated that all the four MAbs reacted strongly with RSMV in crude extracts from RSMV-infected rice tissues or in homogenates from RSMV viruliferous leafhoppers (Fig. 2A, 2B). No positive reaction was obtained when crude extracts from a healthy rice plant or from the RGDV-, RSV-, RBSDV- or SRBSDV-infected rice plants, or from non-viruliferous leafhoppers were used for ACP-ELISA (Fig. 2A, 2B). Western blot assays were also performed to determine the specificities of the four anti-RSMV MAbs. Results showed that all the four MAbs detected a protein band of ~60 kDa in crude extracts from RSMV-infected rice plants or RSMV viruliferous leafhoppers, but not in the crude extracts from healthy rice plants or from non-viruliferous leafhoppers (Fig. 2C, 2D). Based on the estimated molecular weight, we decided that the detected protein is the nucleocapsid protein of RSMV (Fig. 2C, 2D).
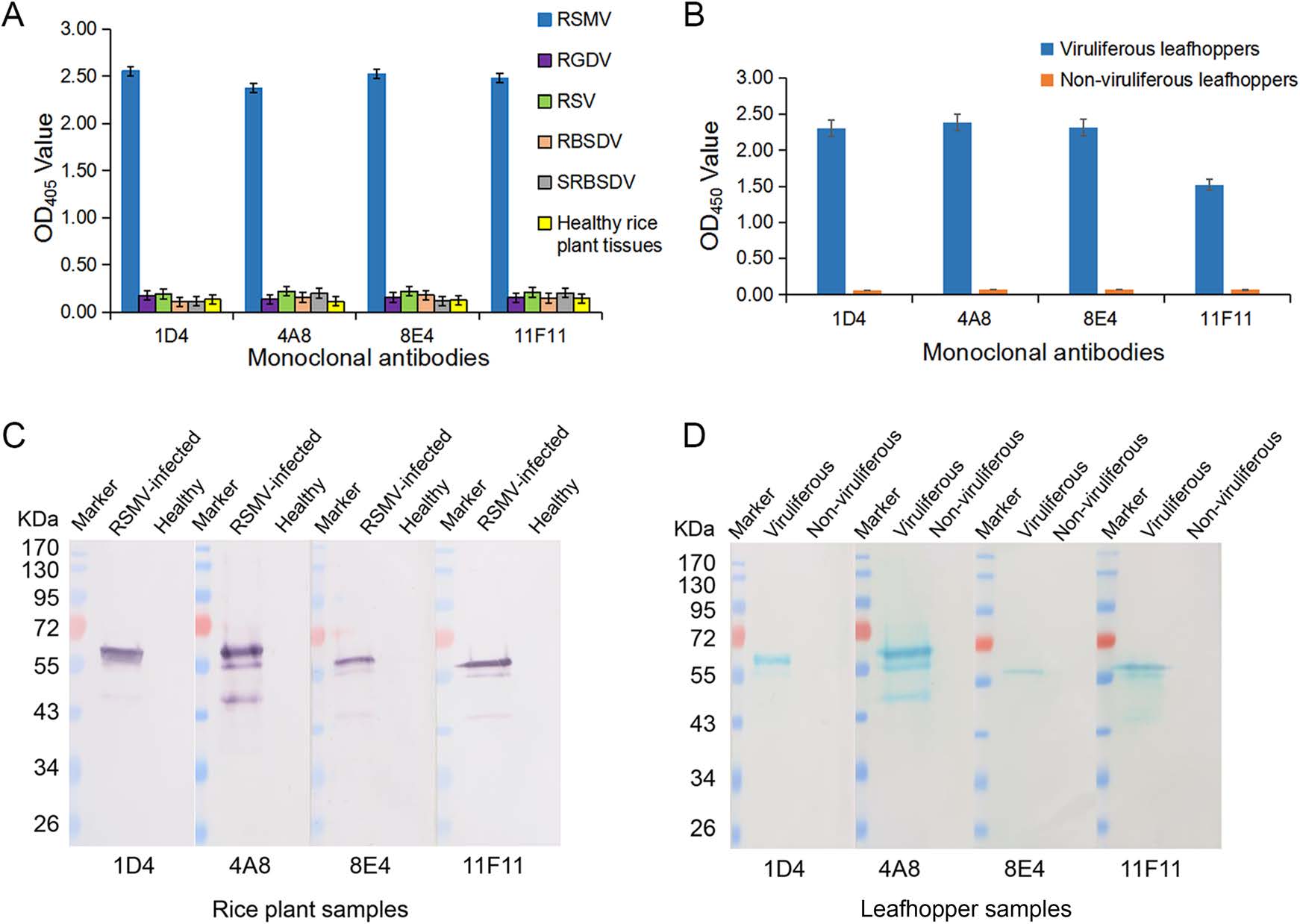
Figure 2. Specificities of the four RSMV MAbs in ACP-ELISA and Western blot. A Specificity analyses of the MAbs through ACP-ELISA. Crude extracts were prepared by grinding tissues from a healthy rice plant, or from the RSMV-, RGDV-, RSV-, RBSDV- or SRBSDV-infected rice plants in coating buffer at a 1:30 ratio (w/v, g/mL). These crude extracts were tested for RSMV infection using the four anti-RSMV MAbs diluted 1:7000 (v/v) in PBST? M. The results are shown as the mean ± standard deviation (SD) from three biological replicates. B RSMV viruliferous and non-viruliferous leafhoppers were homogenized individually in coating buffer, and tested for RSMV infection through ACP-ELISA using three biological replicates per treatment. C Specificity analyses of the four MAbs were done using crude extracts from a RSMV-infected or a healthy rice plant through Western blot. D Specificity analyses of the four MAbs were done using RSMV viruliferous and non-viruliferous leafhopper homogenates through Western blot. Lanes loaded with a protein marker are indicated. Dilutions of the four MAbs were the same as described for ACP-ELISA.
-
The optimal working dilutions of the four MAbs and the AP-conjugated or the HRP-conjugated goat anti-mouse IgG secondary antibody were determined using the phalanx tests. Results from three independent phalanx tests indicated that RSMV could be reliably detected in the infected rice crude extracts and in viruliferous leafhopper homogenates with the four MAbs diluted at 1:7000 (v/v) and the AP-conjugated or the HRP-conjugated goat anti-mouse IgG diluted at 1:8000 (v/v). Cross reaction assay demonstrated that the ACP-ELISA developed in this study gave strong and specific reactions with the RSMV-infected rice plant crude extracts and RSMV viruliferous leafhopper homogenates (Fig. 2A, 2B). No positive reaction was observed when the crude extracts from the RBSDV-, SRBSDV-, RSV- or RGDV-infected or a healthy rice plant, or from a non-viruliferous leafhopper were tested. Serial two-fold dilution assays using crude extracts from a RSMV-infected or a healthy rice plant revealed that this ACP-ELISA was able to detect RSMV in infected crude extracts diluted at 1:2, 621, 440 (w/v, g/mL) using MAb 1D4, 1:5, 242, 880 using MAb 4A8, 1:10, 485, 760 using MAb 11F11 and 1:20, 971, 520 using MAb 8E4, respectively (Fig. 3A). When individual RSMV viruliferous leafhoppers were used, this assay could detect RSMV in the homogenates diluted at 1:307, 200 (i.e., one leafhopper in 307, 200 μL of PBS) using MAb 4A8, 8E4, 11F11, or 1D4 (Fig. 3B). These results indicate that these ACPELISAs are highly sensitive and specific for detections of RSMV infection in rice plant and leafhopper crude extracts.
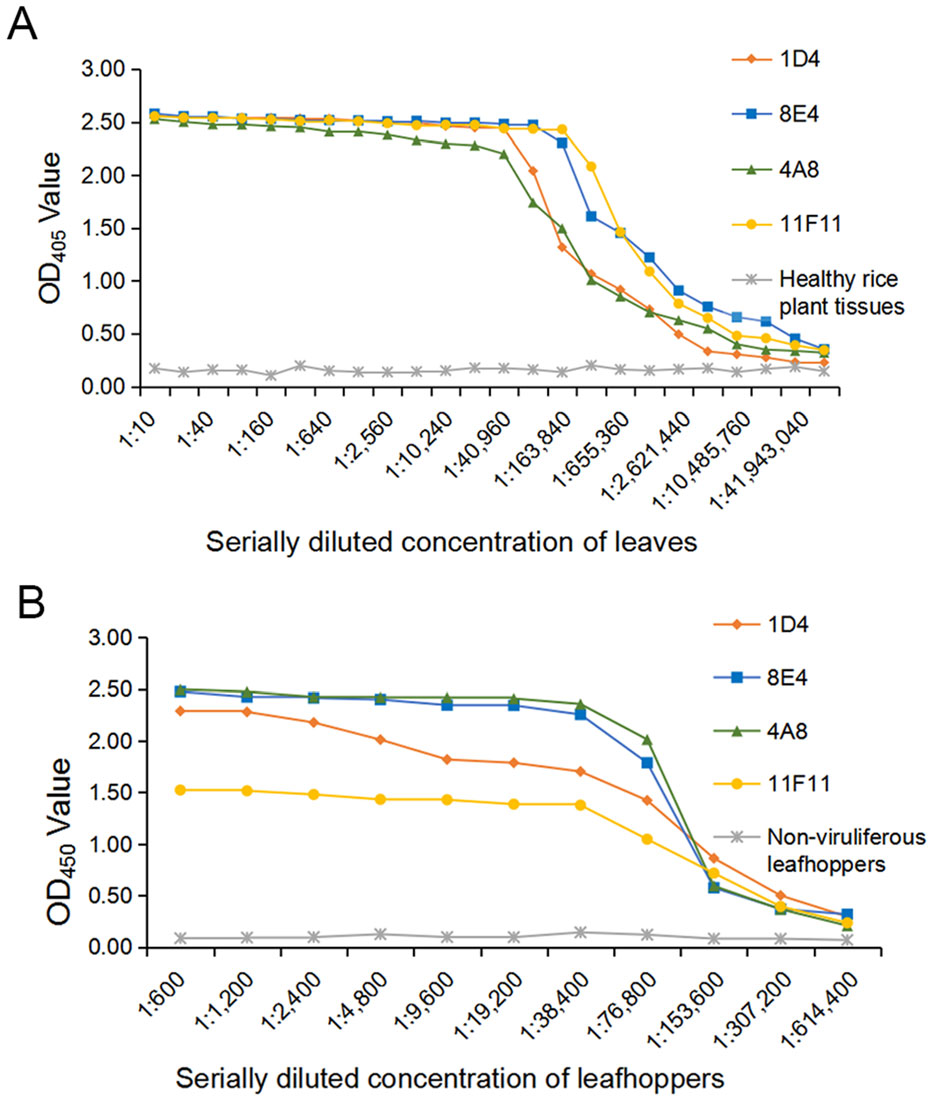
Figure 3. Sensitivity analyses of MAbs through ACP-ELISA. A A crude extract from a RSMV-infected rice plant and a crude extract from a healthy rice plant were separately diluted in a coating buffer, starting from 1:10 to 1:83, 886, 080 (w/v). These samples were tested for RSMV infection through ACP-ELISA using the four MAbs followed by the AP-conjugated goat anti-mouse IgG second antibody. The OD405 values were read at 30 min after the addition of substrate at room temperature. The presented mean values were from three independent assays. B Homogenates from RSMV viruliferous or nonviruliferous leafhoppers were diluted from 1:600 to 1:614, 400 (one insect/μL of coating buffer) and then tested for RSMV infection as described in A.
-
Based on the results of phalanx tests, we used 1:5000 diluted anti-RSMV MAbs and 1:8000 diluted AP-conjugated or HRP-conjugated goat anti-mouse IgG secondary antibody for the Dot-ELISA and Tissue print-ELISA. Crude extracts from RSMV-, RGDV-, RSV-, RBSDV- or SRBSDV-infected or healthy rice plants, or homogenates from RSMV viruliferous or non-viruliferous leafhoppers were blotted onto nitrocellulose membranes for Dot-ELISA. Results showed that strong purple color reactions were developed on the dots from the RSMV-infected rice plants (Fig. 4A). No purple color reaction was observed on the dots from the RGDV-, RSV-, RBSDV- or SRBSDV-infected rice plants or from the healthy rice plants (Fig. 4A). Same results were obtained for the RSMV viruliferous or non-viruliferous leafhopper samples (Fig. 4A). Results of sensitivity assays indicated that using Dot-ELISA, RSMV could be reliably detected in the dots made with 1:327, 680 (w/v, g/mL) diluted RSMV-infected crude extract (MAb 4A8 and 11F11), 1:163, 840 diluted crude extract (MAb 8E4) or 1:81, 920 diluted crude extract (MAb 1D4) (Fig. 4B). For RSMV viruliferous leafhoppers, the virus could be detected in the dots made with 1:163, 840 (individual leafhopper/μL) diluted homogenate (MAb 4A8), 1:81, 920 diluted homogenate (MAb 8E4) or 1:40, 960 diluted homogenate (MAb 1D4 and 11F11) (Fig. 4B, 4C).
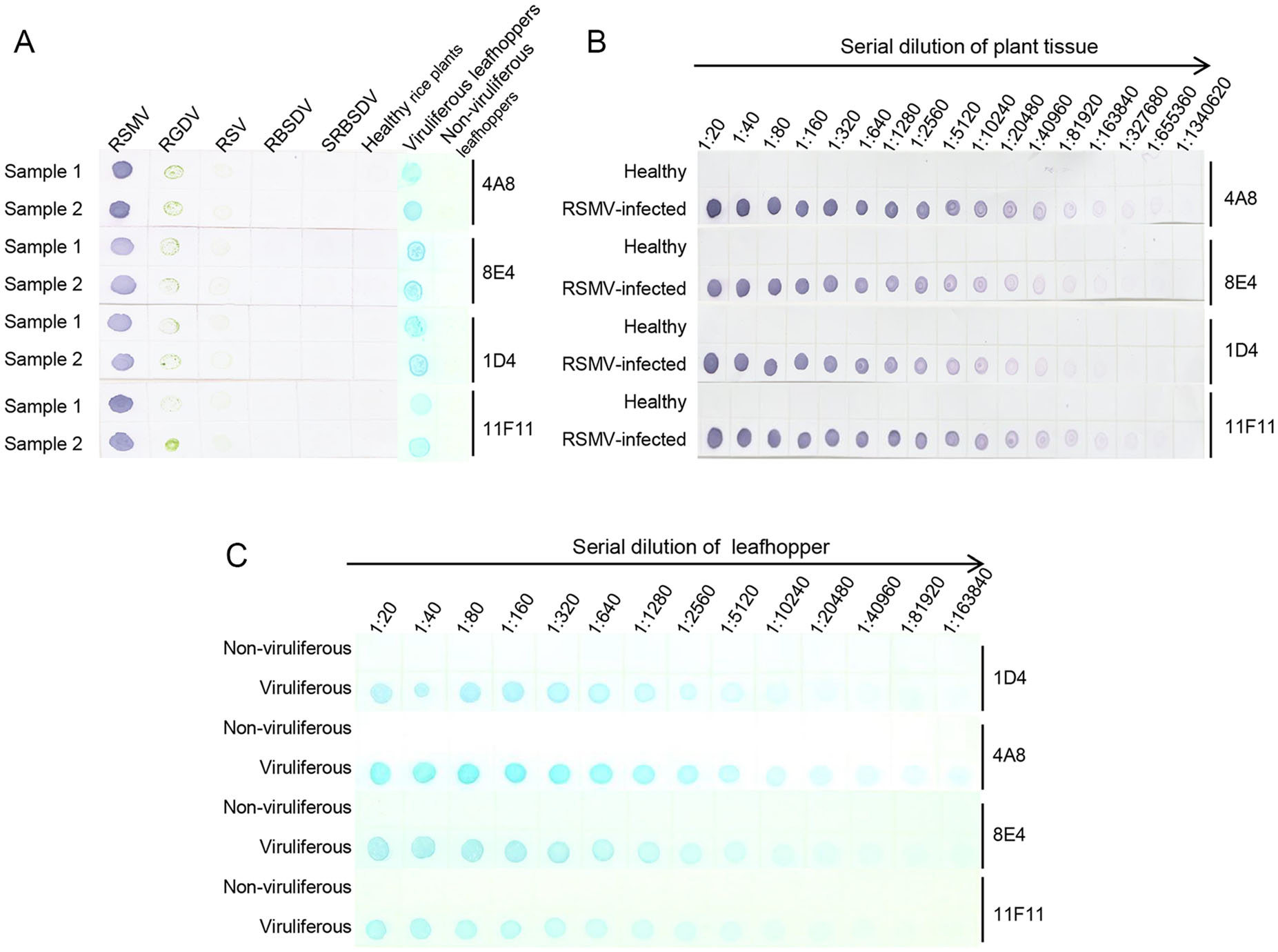
Figure 4. Specificity and sensitivity analyses of the developed dotELISAs. A Specificity analyses of the dot-ELISAs. RSMV, RGDV, RSV, RBSDV, SRBSDV indicate rice crude extracts from the RSMV-, RGDV-, RSV-, RBSDV- or SRBSDV-infected rice plants, respectively. Samples from RSMV viruliferous or non-viruliferous leafhoppers are also indicated above the blot. MAbs used in this study are indicated on the right side of the blots. Purple color and blue color indicate RSMV positive reactions, after addition of NBT/BCIP or TMB substrate. B Sensitivity analyses of the dot-ELISAs for rice samples. Crude extracts from a healthy rice plant or a RSMV-infected rice plant were diluted separately from 1:20 to 1:1, 340, 620 in PBS prior to use. Purple color indicated a positive reaction, after addition of NBT/BCIP substrate. C Sensitivity analyses of the dot-ELISAs for leafhopper samples. Homogenates from an RSMV viruliferous or a non-viruliferous leafhopper were diluted from 1:10 to 1:163, 840 prior to use. Blue color indicated a positive reaction, after addition of TMB substrate.
To determine if Tissue print-ELISA could also be used to detect RSMV infection in rice plants, stems of healthy rice plants and the RSMV-, RGDV-, RSV-, RBSDV- or SRBSDV-infected rice plants were cut and printed individually on nitrocellulose membranes. The membranes were probed with MAbs followed by the AP-conjugated goat anti-mouse IgG secondary antibody. After addition of substrate, strong purple reactions were observed on the prints made with the RSMV-infected rice stems. No purple reaction was observed on the prints made with the RGDV-, RSV-, RBSDV- or SRBSDV-infected rice stems, or with the healthy rice stem (Fig. 5).
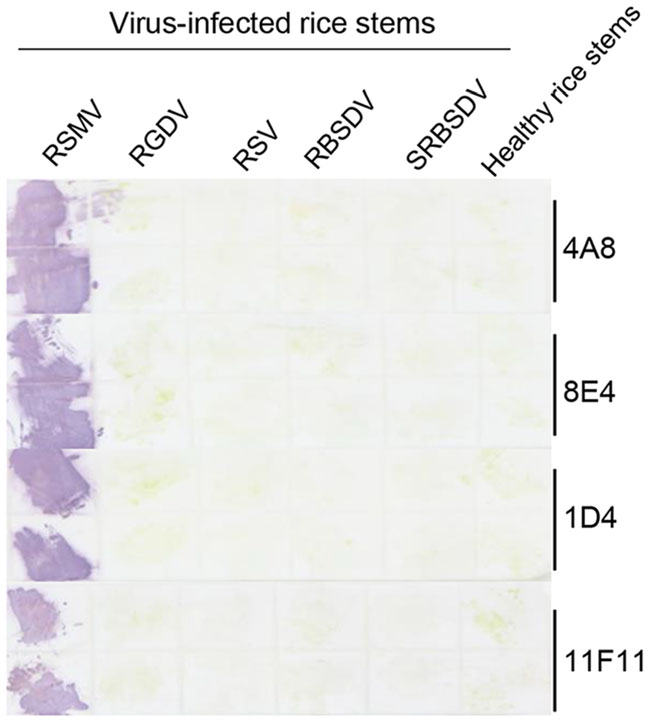
Figure 5. Detection of RSMV infection through the Tissue print-ELISA. Column 1 to 5 had the prints from the RSMV-, RGDV-, RSV-, RBSDVor SRBSDV-infected rice stems. Each treatment had two stem prints (up and down). Prints made with healthy rice stems were used as negative controls. The blot was probed with anti-RSMV MAbs followed by an AP-conjugated goat anti-mouse IgG secondary antibody. Purple color reactions indicated the presence of RSMV in the prints.
-
Because MAb 4A8 showed the highest sensitivity for RSMV detection in above assays, we used it to detect the virus in 33 rice plants and in 16 leafhoppers collected from rice fields in 2018 through ACP-ELISA, Dot-ELISAs and Tissue print-ELISA, respectively. Those three assays took 3–4 h to get the test results, and the results from the three assays indicated that 19 of the 33 rice samples were RSMV-positive (Fig. 6A–6C). Results of ACP-ELISA and Dot-ELISA also showed that 5 of the 16 leafhoppers were RSMV-viruliferous (Fig. 7A, 7B). To validate these serological assay results, we tested the same rice and leafhopper samples by RT-PCR using RSMV P3 gene specific primers. The RT-PCR result was the same as that obtained through the serological assays. All the RSMV positive samples gave a specific PCR product band at approximately 500 bp (Fig. 6D, 7C). DNA sequencing and blast searching showed that the 24 RT-PCR products were all from the RSMV P3 gene. Sequence alignment with the known RSMV P3 gene sequences retrieved from the GenBank showed that the PCR-products of RSMV-infected samples obtained in this study shared 99% sequence similarities with the known RSMV P3 gene sequences.
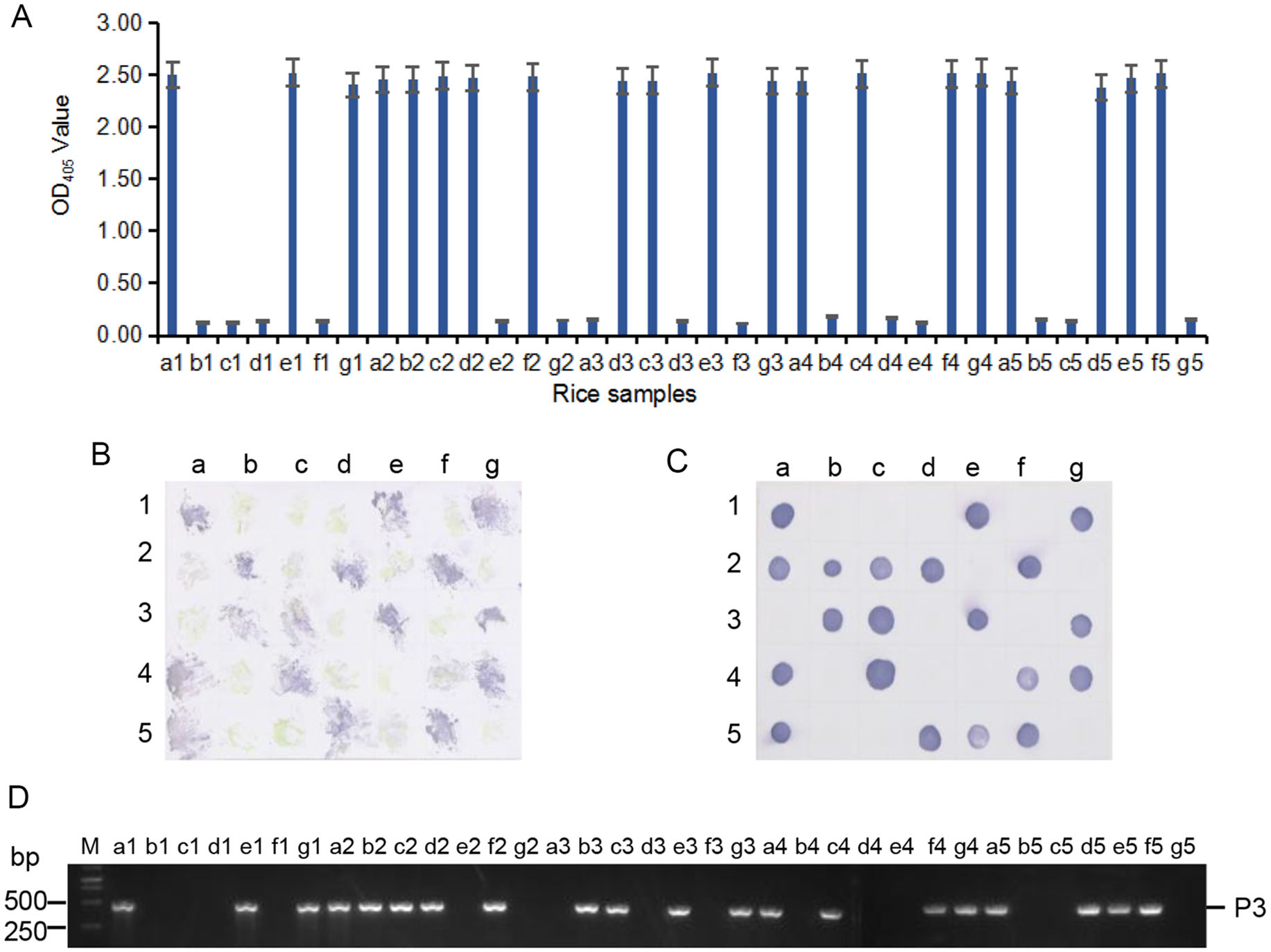
Figure 6. Detection of RSMV infection in the field-collected rice samples through ACP-ELISA (A), Tissue print-ELISA (B), Dot-ELISA (C) and RT-PCR (D). Samples labeled as a1 to a5, b1 to b5, c1 to c5, d1 to d5, e1 to e5, f1 to f4, g1 to g4 were 33 field-collected rice samples. Samples labeled as f5 and g5 were from a RSMV-infected and a healthy rice plant, respectively, and were used as a positive and a negative control. MAb 4A8 was used in these serological assays. Lane M in (D) was loaded with a 1-kb DNA marker. All the RSMV positive samples gave a specific PCR product band at approximately 500 bp in (D).
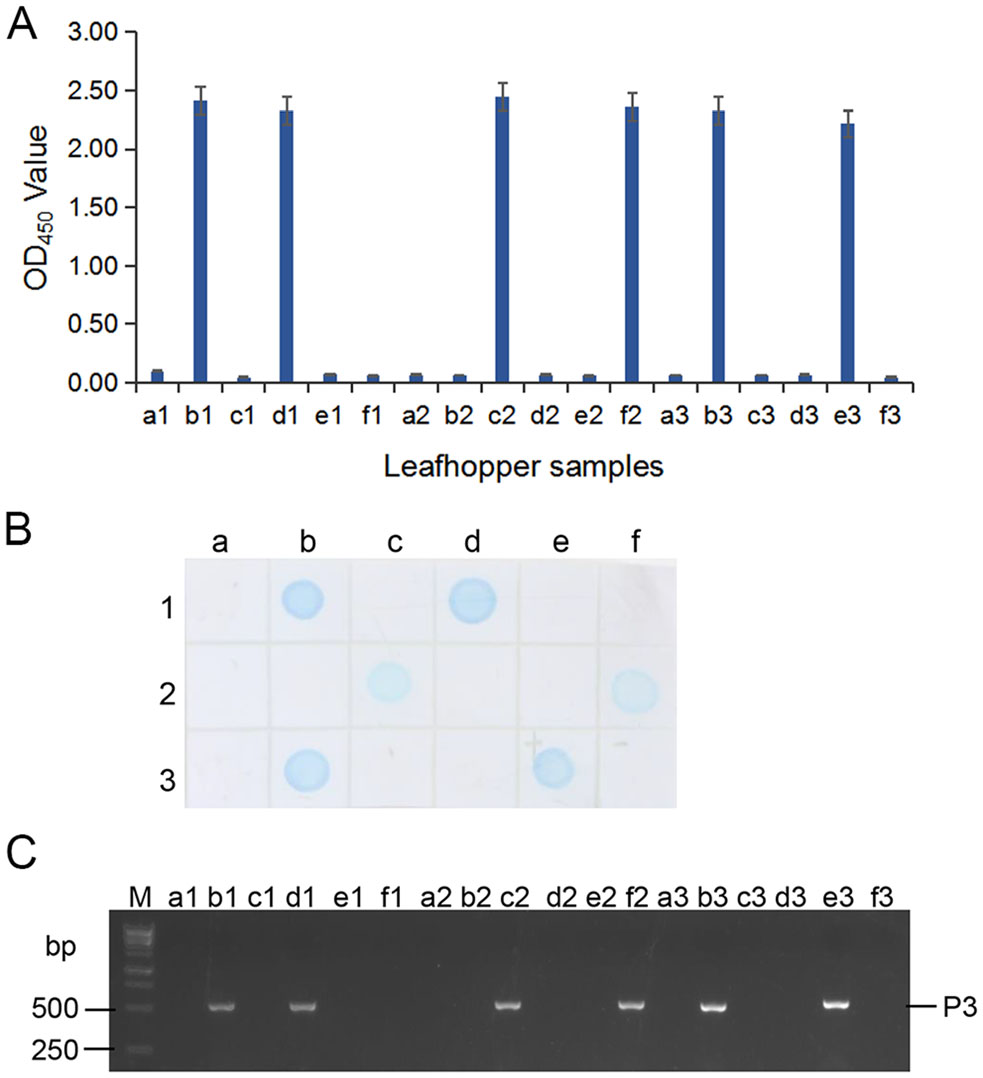
Figure 7. Detection of RSMV infection in the field-collected leafhoppers through ACP-ELISA (A), Dot-ELISA (B) and RT-PCR (C). Samples labeled as a1 to a3, b1 to b3, c1 to c3, d1 to d3, e1, e2, f1 and f2 were from 16 field-collected leafhoppers. Sample labeled as e3 and f3 were from a RSMV viruliferous and a non-viruliferous leafhopper, respectively, and were used as a positive and a negative control. Lane M in (C) was loaded with a 1-kb DNA marker. All the RSMV positive samples gave a specific PCR product band at approximately 500 bp in (C).
Virus Purification
Production and Characterization of Anti-RSMV MAbs
ACP-ELISA for RSMV Detection
Dot-ELISA and Tissue Print-ELISA for RSMV Detection
Detection of RSMV Infection in the FieldCollected Samples Through ACP-ELISA, Tissue Print-ELISA, Dot-ELISA and RT-PCR
-
RSMV is an important rice cytorhabdovirus originally discovered in rice fields in the Guangdong Province, China, in 2015 (Yang et al. 2017a). RSMV causes yellow stripes or mosaics in rice leaves and twisted leaf tips (Yang et al. 2017a). Because symptoms caused by RSMV in rice are very similar to the symptoms caused byRSV, it is difficult to determine RSMV infection through symptom observations. Moreover, many rice viruses do not induce visible symptoms in the early infection stages (Wang et al. 2009). Thus, current identification methods for RSMV are mainly TEM and RT-PCR (Yang et al. 2017a, Yang et al. 2017b, Yang et al. 2018). Because these two methods require expensive instrument and reagents, they are not suitable for large-scale field surveys. Compared with these two methods, serological assays are simple, fast and cost-effective, suitable for high throughput tests. Unfortunately, no RSMV-specific antibody or serological methods for RSMV detection have been reported prior to this study.
To establish a reliable and fast serological assay for RSMV detection, we first generated four hybridoma cell lines secreting highly sensitive and specific RSMV MAbs through the hybridoma technique. We then showed that these four MAbs can react strongly with RSMV in crude extracts from RSMV-infected rice plants or in homogenates from RSMV viruliferous leafhoppers through ACP-ELISA, Dot-ELISA or Tissue print-ELISA. No false positive were observed with the samples from the RSV-, RGDV-, RBSDVor SRBSDV-infected rice plants or from healthy rice plants. Similarly, no false positive was found with homogenates from non-viruliferous leafhoppers. Through ACP-ELISA and Dot-ELISA, we have found that RSMV can be readily detected in 1:20, 971, 520 (w/v, g/mL, in ACP-ELISA) or 1:327, 680 (in Dot-ELISA) diluted crude extracts from RSMV-infected rice plants, or in 1:307, 200 (individual leafhopper/μL, in ACP-ELISA) or 1:163, 840 (in Dot-ELISA) diluted leafhopper homogenates. Our serological assay results are consistent with the results of RT-PCR, confirming the specificities and sensitivities of the MAbs and the reliabilities of the three serological methods. We consider that these four anti-RSMV MAbs and the three newly developed serological methods are very useful for RSMV epidemiological studies, resistant rice breeding and establishment of control strategies for this virus.
-
Project was supported by the Ministry of Agriculture of China (No. 2016ZX08009003-001), the National Key Research and Development Program of China (No. 2016YFD0300706), the National Natural Science Foundation of China (No. 31571976), and the Earmarked Fund for China Agriculture Research System (No. nycytx-001).
-
LG and JW prepared the MAbs and developed the serological assays. RC performed RT-PCR and field sample detections. JH participated in the study design and wrote part of the manuscript. XZ and JW conceived the study and finalized the manuscript. All authors have read and approved the final manuscript.
-
All authors declare that they have no conflict of interest.
-
The animal experiments were performed in accordance with the Principles of the Helsinki accords and approved by the Animal Experimentation Ethics Committee of Zhejiang University, Hangzhou, China.














 DownLoad:
DownLoad: