-
White spot syndrome disease is caused by the White spot syndrome virus (WSSV). It was first reported in Taiwan in 1993 and rapidly emerged as a major viral disease of shrimp worldwide (6). The virus can infect many other marine and freshwater crustaceans,including crayfish and crabs (13, 18). WSSV is now one of the most serious viral diseases known to the shrimp farming industry,causing heavy economic losses.
WSSV is a large enveloped double-stranded DNA virus and ovoid-to-bacilliform in shape. It is about 275 nm in length and 120 nm in width with a tail-like appendage at one end (32). The complete genome sequence of WSSV (290-305 kb) contains approximately 180 putative open reading frames (ORFs) (26, 33). At least 39 structural proteins are currently known. VP26,VP36A,VP39A,and VP95 were all identified as tegument proteins,VP19,VP28,VP31,VP36B,VP38A,VP51B,VP53A were identified as envelope proteins,and VP664,VP51C,VP60B,VP15 were classified as nucleocapsid proteins (24).
Thus far,several laboratory methods have been established to detect WSSV. These include transmis-sion electron microscope (TEM),histological exa-mination of hematoxylin and eosin [H & E]-stained tissues from moribund shrimp by light microscopy (28) ,polymerase chain reaction (PCR) (10, 14, 19) ,in situ hybridization using DNA probe (9) ,and immunological methods (7, 17, 29). Each of these methods has particular advantages and disadvantages in terms of sensitivity,specificity,cost and convenience. Immunochromatographic assays were first described in the late 1960s. It is a rapid,efficient,sensitive method. Over the past decade many immunochromatographic assays have been reported for the detection of infectious diseases (1, 4, 8, 31) ,cancer (11) ,cardiovascular problems (20, 22) ,and illicit drugs (2). Other promi-sing areas for the use of such assays are drug monitoring (30) ,food safety (5) ,and veterinary medicine (15).
Immunochromatographic assays have several diffe-rent formats. In this study,we developed a membrane-based lateral-flow immunoassay (LFIA) to detect WSSV. Our device adopted a "sandwich" format,in which the unconjugated anti-VP (28+19) antibodies were used as a capture antibody and the colloidal gold-labeled antibody was used for detection. This method can detect WSSV rapidly,expediently and efficiently and may be used directly on shrimp farms as a "pond-side test" once a more user-friendly version is developed.
HTML
-
The recombinant plasmids vector pET-22b (+)-VP (19+28),previously constructed in our laboratory (16) ,was transformed into E. coli Origami (DE3) pLysS strain. The transformed strain was induced by 0.6 mmol/L isopropyl-beta-D-thiogalactopyranoside(IPTG),and continued to grow for 4 h in LB medium (1% tryptone,0.5% yeast extract,1% NaCl,pH 7.4) containing 100 μg/mL ampicillin,15 μg/mL kanamycin,34 μg/mL chloramphenicol at 37℃. The culture was centrifuged at 4 000g (20min,4℃). Cells were suspended in lysis buffer (10 mmol/L imidazole,300 mmol/L NaCl,50 mmol/L NaH2PO4,pH 8.0). The suspensions were freeze-thawed at -20 ℃ several times,and then sonicated on ice for 30 s. The sonicated sample was centrifuged at 14 000 g (30 min,4℃) and analysed by SDS-PAGE.
The recombinant fusion protein VP(19+28) was purified on a Ni-NTA Superflow chromatography column (Qiagen) following protocols provided by the manufacturer and then analyzed by SDS-PAGE. The purified protein was concentrated and adjusted with 0.9% NaCl by ultrafiltration (Millipore). For immunization,200μg of the purified protein in 500μL was mixed with equal volume of Freund's complete adjuvant (Sigma Inc,St. Louis,MO,USA) and injected hypodermically into a New Zealand rabbit. The rabbit was boosted twice at 2-week intervals using the same condition as the first injection except for the replacement of the Freund's complete adjuvant with the Freund's incomplete adjuvant (Sigma). One week after the last immunization,the rabbit was exsanguinated to collect the antiserum.
The antiserum was purified on a Protein A CL-4B chromatography column (Amersham) and analysed by SDS-PAGE. The purified IgG was concentrated and buffered-changed to 10 mmol/L Tris-Cl (pH 8.0) by ultrafiltration (Millipore).
-
All glassware were scrupulously cleaned. Glass and plastic containers and stirrers were cleaned in aqua regia,thoroughly washed in deionized water,and siliconized. All reagents were prepared with deionized water and filtered with 0.22 μm filter immediately before use. Colloidal gold was prepared by the trisodium citrate method (12). A solution containing 100 mL of a 0.01% (w/v) HAuC14 (Sigma) solution was prepared,and heated to boiling temperature then 2.0 mL of a 1% (w/v) trisodium citrate solution was added. The solution was boiled for a further 15 min,then cooled to RT,and the pH adjusted to 9.0 with 0.1mol/L K2CO3.
Prior to conjugation,the anti-VP (19+28) polyclonal IgG (1.25 mg of protein) were diluted to 5 mL with 10mmol/L Tris-Cl (pH 9.0),and added into 100 mL of the colloidal gold solution with agitation. After 10 min,10% BSA (w/v) was added to a final concentration of 1%. The solution was then centrifuged at 15 000g for 1 h at 4℃. After discarding the supernatant,the pellet was washed twice with 10 mmol/L Tris-Cl (pH 9.0),and resuspended in a final volume of 10 ml phosphate-buffer (50 mmol/L,pH 7.5) containing 1% BSA.
-
The fiberglass membrane (Millipore,USA) was dipped in a colloidal gold-IgG conjugation mixture for 30 min and the membranes were dried at 37℃ for 30 min.
Nitrocellulose -backed membranes (Millipore) were cut into 3-cm by 5-mm strips. The purified anti-VP (19+29) polyclonal IgG (1.25mg/mL) was sprayed as the Test line,and goat anti-rabbit IgG (2.0mg/mL,Pierce,USA) was sprayed at a 5-mm distance from the test line as the Control line. The membranes were allowed to air dry at room temperature (RT),then bloc-ked by incubating with 1% casein enzymatic hydrolysate (Sigma) for 1h at 37℃ and dried again at RT.
To assemble the strips (Fig. 1),materials including the sample pad (Glass fiber,Pall,USA),conjugate pad,NC membrane,and absorbent pad (Cotton linters,Pall) were attached as slightly overlapping strips to plastic backing,from which 5-mm strips were cut. The strips were relatively stable if they remained thoroughly desiccated and could be stored with dehumidification in cylinders or in foil pouches at ambient temperatures until use.
-
A series of samples were prepared,applied to the test strips,and chromatography was allowed to proceed for 15 min at RT. The test strips were observed for the presence of pinkish purple lines. Distilled water,50 mmol/L PB (pH7.0),Origami (DE3) pLysS culture,and healthy shrimp samples were tested as negative controls,and purified VP (19+28) recombinant protein,WSSV viral antigen,and infected shrimp samples were tested as positive controls.The sensitivity was determined using purified VP (19+28) in the concentration of 10 μg/mL,groups: 100 μL for the moribund group (group A); 200μL for the lethal group (group B); and 200μL PB (50mmol/L,pH7.0) for the healthy group (group C). Five shrimps were picked randomly from each group hepatopancreas samples were abraded and suspended in 50 mmol/L PB (pH7.0). The processed samples were then used for testing as described above. At the same time,DNA was extracted from each sample PRC) and analyzed by PCR using the following primers: vp19-FW 5'-ATG GCCACCACGA CTAA-CAC-3',vp19-RV 5'-CGAAAGGGA AACG TCA-GA AA-3'. The 20 L-PCR reaction contained 50 mmol/L KCl,10 mmol/L Tris-HCl (pH 9.0),1.5 mmol/L MgCl,0.4 mmol/L dNTP,30pmol/L of vp19-FW,30pmol/L of vp19-RV,2 μL of template,and 4 U Taq polymerase. The PCR reaction was initiated by heating the mixture to 95℃ for 10 min followed by 30 cycles of 60 s at 94℃,30 s at 55℃ and 30 s at 72℃ with a final extension of 10 min at 72℃
In order to test the stability of the strips,the same samples were assayed by the strips which were stored at 4℃ or RT for 30 days,respectively.
1.1. Production and purification of antibodies
1.2. Colloidal gold preparation and conjugation
1.3. LFIA strip preparation
1.4. Assay procedure
-
Analysis by SDS-PAGE indicated a 41 kDa protein band in the pET-22b (+)-VP (19+28)/Origami (DE3) pLysS (Fig. 2,lanes 2),but not in the non-induced samples (Fig. 2. lanes 1,3) or in the induced pET-22b (+)/Origami (DE3) pLysS (Fig. 2. lanes 4,5). After sonication and centrifugation,both supernatant and the insoluble fraction were analyzed by SDS-PAGE (Fig. 2. lane 6,7). The 41 kDa protein band was observed only in the supernatant.

Figure 2. SDS-PAGE analysis of expressed fusion protein. The concentration of separating gel was 10% and visualized by coomassie brilliant blue R250 staining. Lanes: 1,pET-VP (19+28) before induction; 2,pET-VP (19+28) induced; 3,pET-VP (19+28) non-induced; 4,pET-22b(+) non-induction; 5,pET-22b(+) induced; M,molecular weight standard; 6,supernatant after sonication; 7,insoluble fraction after sonication. Arrow indicates the location of recombinant VP (19+28).
The VP (19+28) fusion protein from the soluble fraction was purified on a Ni-NTA Superflow column and analyzed by SDS-PAGE (Fig. 3. lanes 1~4). The eluted fractions revealed a single band with an apparent mo-lecular weight of 41 kDa,which has the same mobility as the induced recombinant protein from the crude culture.
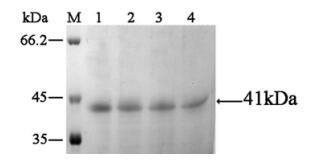
Figure 3. SDS-PAGE analysis of purified recombinant protein. The concentration of separating gel was 10% and visualized by silver staining. Lanes 1-4,factions of purified protein eluted from the Ni-NTA column; M,molecular weight standards.
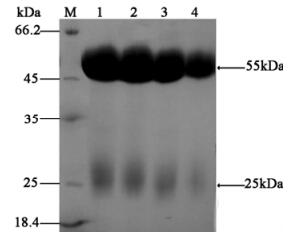
The anti-VP (19+29) IgG were isolated and purified from VP (19+28) antiserum using a Protein-A column. When the purified IgG was analyzed,both the heavy and light chains were visible and no other contamina-ting proteins appeared to be present,indicating that the affinity purification had worked efficiently (Fig. 4).
-
For specificity testing,four negative control samples and three positive control samples were applied to the strips and the results are presented in Fig. 5A. For samples containing distilled water,PB,Origami (DE3) pLysS culture,and healthy shrimp samples,only the control lines showed a response up (Fig. 5. 1~4). On the other hand,when VP (19+28) recombinant protein,WSSV viral antigen and infected shrimp samples were applied,both the control and test lines could be observed (Fig. 5. 5~7).

Figure 5. Specificity tests of assay strips. 1,Distilled water; 2,PB; 3,Origami (DE3) pLysS culture; 4,Healthy shrimps; 5,VP (19+28) recombinant protein; 6,WSSV; 7,Infected shrimps.
Similar results were obtained when strips stored at 4℃ or RT were used,indicating the pre-fabricated strips were stable at both temperatures tested (data not shown). For sensitivity testing,the purified recombinant protein VP (19+28) serial dilutions from 10 mg/mL to 5 ng/mL were applied to the strips,and the lowest detectable concentration was at 10 ng/mL (Fig. 6).

Figure 6. Sensitivity tests of assay strips. VP (19+28) recombinant protein concentration: 1,10μg/mL; 2,1μg/mL; 3,100ng/mL; 4,20ng/mL; 5,10ng/mL; 6,5ng/mL.
For comparison of the detection sensitivity and specificity between LFIA and PCR,all samples were tested in both assays at the same time. Results are summari-zed in Table 1 and Fig. 7 (A for LFIA and B for PCR).

Table 1. Comparison of LFIA and PCR (number of positive/total number tested)
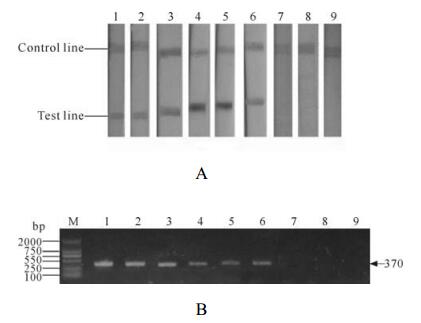
Figure 7. LFIA (A) and PCR (B) anlaysis of different tissues from the moribund,dead and healthy shrimps. Samples 1,body juices of healthy shrimps; 2 gills of healthy shrimps; 3,hepatopancreas of healthy shrimps; 4,body juices of moribund shrimps; 5 gills of moribund shrimps; 6,hepatopancreas of moribund shrimps; 7,body juices of dead shrimps; 8 gills of dead shrimps; 9,hepatopancreas of dead shrimps.
2.1. Preparation of antibodies
2.2. Assay development and testing
-
WSSV is an enveloped virus,which has five major proteins. VP28 and VP19 were identified as envelope pro-teins. VP28 was demonstrated to play an important role in the systemic WSSV infection in shrimp. VP19 may also be involved in systemic infection,either alone or in coope-ration with VP28 (27). Since these major WSSV proteins are not glycosylated (25) ,the E. coli expression system was chosen to express this fusion protein.
Origami (DE3) strains carry the trxB mutation and the gor mutation,which have the potential to enhance disulfide bond formation and ultimate solubility and activity/ antigenicity (3). Previous studies have shown that expression in Origami (DE3) yielded 10-fold more active protein than in another host even though overall expression levels were similar (23). In this study,although the protein is not active,it is believed that the correct folding of the protein may increase its antigenicity/immunogenicity when used as an immunogen for antibody production in rabbits. For this reason,the Origami (DE3) pLysS strain was chosen for expression.
Major advantages of the rapid lateral-flow immunoassays over other testing formats include rapidity,simpli-city,and the need for minimal training of personnel. This assay can be performed virtually anywhere,in laborato-ries,in office,or in the field. The strips we developed are very efficient. It can identify WSSV infection from many different tissues of shrimps. Particularly,it can detect WSSV form the shrimps′body juice directly,which makes pretreat-ment unnecessary and will greatly enhance the practicality of using such assays in the field,e.g.,directly on shrimp farms. It is important to emphasize that our prototype assay was able to detect WSSV infection not only in dead shrimps,but also in moribund shrimps. This is very important in disease response. Rapid and conclusive diagnosis of WSSV infection in moribund shrimps would provide a timely warning alarm so that the farmers can take appropriate measures to prevent further spread of the disease at an early stage.
In conclusion,we have established a rapid,simple and efficient method to detect WSSV. It can identify the infection of WSSV and can be used in disease response,and maybe also in disease prevention if used to survey the prevalence of white spot syndrome disease. To date,there has been neither highly effective drug nor good treatment available for this viral disease. Therefore,at present,early diagnosis is the only viable option to fight this widespread disease. Development of a hand-held test like ours will make significant contribution to the control of this disease. Wide application of such a test will also encourage the implementation of preventative or control measures.














 DownLoad:
DownLoad: