-
Generally, cell death occurs by necrosis and apoptosis. Necrosis is the result of immediate, physically damage to cells causing swelling, rupture and release of cellular contents. Apoptosis, on the other hand, is a regulated cascade of physiological response culminating in cell death. Apoptosis is recognized by cell shrinkage followed by plasma membrane blebbing and release of apoptotic bodies. In vivo, the apoptotic bodies undergo phagocytosis by surrounding cells. In culture, where phagocytosis is limited, secondary breakdown of apoptotic bodies occurs releasing cellular constituents into the medium.
Although the apoptotic program is highly complex and the cellular events involve the activation of many signaling cascades, two main pathways predominate; the mitochondrial-mediated pathway and the cell surface-mediated signal transduction pathway. The mitochondria, which are the energy generators for the cell, also house apoptosis-instigating molecules including cytochrome c, Smac/DIABLO, and AIF (5, 10, 16). These molecules are released in response to apoptotic stimuli such as toxins or DNA damage and promote activation of pro-caspase 9, a principal cysteine-aspartate protease responsible for initiating cellular apoptosis signaling. Caspase 9 as well as other caspases exist in an inactive zymogen pro-caspase state. Proteolytic processing is required to convert the pro-caspase into a proteolytically active caspase molecule. The cell surface-mediated signal transduction pathway is triggered through the binding of ligands to death receptors at the cell plasma membrane, allowing for the recruitment of deathincluding complexes at the cytoplasmic tail of the receptors. The classic model for this process involves binding of the trimeric Fas ligand to the Fas receptor. A conformational change ensues allowing for the recruitment of the proteins FADD and pro-caspase 8 to the cytoplasmic death domain of the receptor. This complex formation leads to caspase 8 activation. The mitochondrial-mediated and cell surface signal transduction pathways converge at the activation of caspase 3. Thus, at the apex of the two apoptosis cascades are the initiator caspases, caspase 8 on the cell surface signaling pathway and caspase 9 in the mitochondrial-mediated pathway. The initiator caspases 8 and 9 in turn activate the downstream effector class of caspases 3, 6 and 7. The activation of these effector caspases leads to the final execution of the cell death program.
Viruses have evolved novel mechanisms to prevent apoptosis of the infected host cell and thereby promote virus multiplication (12, 13). The baculovirus Autographa californica nucleopolyhedrovirus (Ac MNPV) was the first insect virus known to induce and inhibit apoptosis (3). Infection of Spodopera frugiperda Sf-21 cells with the wide-type (wt) AcMNPV results in the production of both budded virus and occluded virus. In contrast, infection with an AcMNPV mutant lacking the P35 gene results in induction of apoptosis with limited budded virus production and no occluded virus production (14). To date, two distinct types of apoptotic suppressors, represented by P35 and the inhibitors of apoptosis (IAPs), have been identified in the invertebrate baculoviruses (2). Baculovirus IAPs were the first members of the IAP protein family to be discovered (4, 11). The best-studied viral IAP, that from the Orgyia pseudotsugata MNPV (OpMNPV), prevents apoptosis in insects and mammals by a mechanism that includes interaction with itself and pro-apoptotic proteins like Reaper, HID and GRIM of Drosophila (1, 7, 9, 17, 18)
SeMNPV has been registered as a biological insecticide to control the target pest(19). Its genome contains two iap genes (20), the IAPs interactaction with Ubiquitin has been reported (22) but their function in vivo is unkown. In this study, we cloned and expressed the SeMNPV iap3 gene in HEK293 cells and demonstrated the anti-apoptotic activity of SeMNPV IAP3 in the mammalian HEK293 cells.
HTML
-
We extracted the SeMNPV genome and used it as the template of PCR. The following primers were used to amplify the iap3 isoform: 5'-TTAGAATTCTATG CAGGTGAACAGCGATG-3', 5'-GAAGGATCCTC AGCACTGACACTACATTTAAAC-3'
The PCR product was cloned into pEGFP-C1 by using the above primers incorporating specific restriction enzyme sites: EcoR I/BamH I yielding pEGFP-iap 3 plasmid. The plasmid constructs were verified by DNA sequencing.
-
Human embryonic kidney (HEK) 293 T cells were maintained at 37℃ and 5% CO2 in Duldecco's minimal essential medium (Invitrogen, Carlsbad, USA) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen, Carlsbad, USA), penicillin and streptomycin. Cultures were maintained in 25 cm2 cell culture flasks (Corning) and passaged every 72 h. Transfections were performed using LipofectAMINE 2000TM (Invitrogen, Carlsbad, USA) as outlined in the manufacturer's protocol. Plasmid DNAs were prepared using the E.Z.N.A.® Endo-free Plasmid Miniprep Kit Ⅱ (Omega, Doraville, USA) according to the manufacturer's protocol.
-
HEK293 cells were plated in 6-well tissue culture dishes (Corning, NY, USA) at a density of 5×104 cells/dish; 24 h after plating, the cell were transtected using LipofectAMINETM to express each of the following: GFP, GFP/IAP3. After transfection, cells were exposed to complete growth medium with cisplatin. Viability data was determined at 12 h internals by counting cells per sample, 4 sample each. Only these cells containing fluorescence were scored for apoptotic morphology.
-
Apoptosis was induced in HEK293 cells with the chemical reagents cisplatin (cis-platinum (Ⅱ) diam-mine dichloride). HEK293 cells were transfected with either pEGFP-iap 3 or pEGFP-C1 and plated in duplicates in 6-well tissue culture dishes at a density of 4×105 cells/well and were allowed to attach for 2 days, forming stably transformed cells. To induce the apoptosis, each well was washed with 0.01 mol/L PBS (pH7.2-7.4) followed by addition of complete growth medium containing 50 μg/mL cisplatin. The trans-formed cells treated with the drug were harvested at 12 h, 24 h, 36 h after treatment respectively, GFP+ Cells were scored for nucler morphology of apoptosis by 4'6-diamidino-2-phenylindole (DAP1) staining and fluorescence microscope. Each time, five viability counts were taken for the cells transfected with pEGFP-iap 3 or pEGFP-C1 respectively, to determine the average viability and standard deviation (21). The percentage of cell survival after treatment with the drug was estimated and the function of SeMNPV IAP3 in mammalian cells was evaluated.
-
For the analysis of apoptosis by DNA laddering in HEK293 cells, the cells were trnsfected with pEGFP-iap 3 or pEGFP-C1 respectively, the transfected cells were plated in 6-well tissue culture dishes. 48 h later, 50μg/mL cisplatin was added to each well. Cells were harvested at 12 h intervals, washed twice with 0.01mol/L PBS, resuspended in lysis buffer (100mmol/L Tris-HCl, pH 8.0 and 20mmol/L EDTA, 0.8% sodium lauryl sacosine) and stored at 4 ℃ for 15 min. Cell lysates were then centrifuged at 13 000 × g at 4 ℃ for 30 min. The supernatants were collected and incubated with 100μg/mL RNase A and 240μg/mL proteinase K at 37℃ for 1 h. Crude DNA prepara-tions were extracted with phenol: chloroform: isoamy-lalcohol (25:24:1), precipitated with 0.1 volume of 3 mol/L sodium acetate and 2.5 volumes of 100% ethanol at −20℃ overnight and centrifuged at 14 000×g for 30 min. The DNA pellets were air-dried and resuspended in TE buffer (10mmol/L Tris-HCl, pH 8.0, 1mmol/L EDTA). The concentration of nucleic acid was determined by UV absorbance at 260 nm and analyzed on 1.8% agarose gels and photo-graphed in an image analyzer Gel Doc 2000 (Bio-Rad, Hercules, CA).
Construction of plasmids
Cell culture and transient expression
Transient viability studies
Anti-apoptosis analysis
DNA fragmentation
-
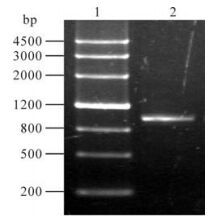
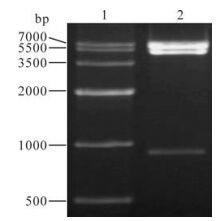
A pair of primers were designed for eukaryotic expression based on the SeMNPV iap3 sequence in GenBank. It was amplified by PCR and the products were analyzed on a 1.0% agarose gel. The expected 1kb fragment was obtained (Fig 1. lane 2). The SeMNPV iap coding sequence was cloned in the EcoRⅠ and BamHⅠ sites of the pEFGP-C1 vector. The recombinant was verified by digested by EcoRI/BamHⅠ (Fig 2. lane 2). In these combinant plasmids, a green fluorescent protein (GFP) gene was fused to the N-terminus of SeMNPV iap2 or iap3. After expression, the GFP is a marker for transfected cells.
-
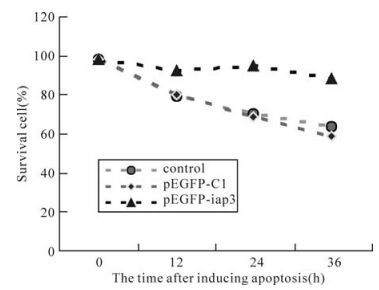
To test the ability of SeMNPV IAP3 to inhibit apoposis, the genetically engineered HEK293 cell lines were exposed to an apoptosis inducing anticancer toxin: cisplatin. The toxin directly binds DNA by forming intraand inter-strand cross links, which cause the DNA to unwind and bind other proteins, thus preventing DNA repair. Cisplatininduced apoptosis has been found to invoke a caspase-3 mediated pathway. The HEK293 tranaformed cells were exposed to 50μg/mL cispatin with subsequent data analysis at 12 h intervals. By 36 h, the GFP control and initial cells were 63.56% and 68.25% viable, respectively, while cell expressing IAP3 were refractory cisplatin-induced death with a viability of 91.07% (Fig. 3). We concluded that in this mammlian cell line, the apoptosis induced by drug was inhibited strongly by SeMNPV IAP3.
-
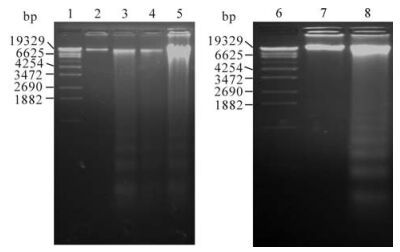
To ascertain if these cells undergo apoptosis after exposure to cisplatin, They were exposed to the toxin, and the genomic DNA was analyzed on agarose gels. Lysates from different time points were run on a 1.8% agarose gels. The addition of ciaplatin to the culture did not cause detectable change in DNA fragmentation until 24 h but was detectable later on (Fig. 4. lane 5 and 8). Controls showed no evidence of laddering (Fig. 4. lane 2 and 7).
Generation of combinant plasmids
The function of SeMNPV IAP3 in stably transformed HEK293 cells
Fragmentation of cellular DNA
-
The IAPs family is a major group of apoptosis regulators that have been shown to directly block caspase activity. We know that SeMNPV has two iap genes, iap2 and iap3. The aim of this study was to genetically inhibit apoptosis in HEK293 cell line by IAP3 of SeMNPV. In order to study the transfection condition and the pattern of protein expression, a green fluorescent protein (GFP) was fused to the N-terminus of SeMNPV IAP3. To determine whether the IAP3 had the function of anti-apoptosis in HEK293 cells, they were exposed to various doses of the toxin cisplatin. Each dose was examined for its ability to cause apoptosis. Apoptosis in HEK293 cells was detected and monitored with time by the detection of DNA laddering as a test for DNA fragmentation. The laddering occurs as a result of clearage of the chromosomal DNA into 180-300 base pair fragments. Optimal DNA fragmentation was observed at 24-36 h post cisplatin exposure.
In this report, the results showed that the SeMNPV IAP3 protects against apoptosis induced by cisplatin eaposure in HEK293 cells. In our previous study, we have forecasted the SeMNPV IAP3 contains BIR domain and RING domains based on its nucleic acid sequence; with two-hybrid system, the interaction beween Ubiquitin and SeMNPV IAP2 or IAP3 has been studied. However, the mechanism that SeMNPV IAP3 how to perform anti-apoptosis in mammalian cells was not clearly. In mammalian cells, the mechanism of SeMPV IAPs worked if is like other mammalian IAPs, such as X-IAP or Survivin done? The SeMNPV IAPs whether could antagonist these mammalian IAPs by competitive inhibition in mammalian cells? Further studies should been done in next.













 DownLoad:
DownLoad: