-
Foot-and-mouth disease is a highly contagious vesicular disease of cloven-hoofed animals. Its pathogen is foot-and-mouth disease virus (FMDV) which is a member of the genus Aphthovirus and of the family Picornavidae and includes seven serotypes: O, A, C, Asia 1, SAT 1, SAT 2 and SAT 3. Asia 1 is widely distributed and very active in the Middle East and Asia (8).
In a mature virus particle, 60 copies of each of the four structural proteins VP1-4 associate to form a capsid which surrounds and protects the genome (7). FMDV serotypes have 86% amino acid (aa) sequence identity to each other; VP1 is more variable and VP2 is relatively conserved (4). FMDV varies greatly at different antigenic sites. Consequently, many FMDV-specific antibodies bind only to homologous FMDV, but not to heterologous FMDV. Animals that have previously been infected with one serotype remain susceptible to the other serotypes (1). This makes FMDV diagnosis by immunological methods more complicated and difficult.
Monoclonal antibodies (mAbs) have been widely used in infectious diseases research, diagnosis and therapy. Compared to the tests based on polyclonal anti-sera, diagnostic tests with mAbs always have higher specificity, accuracy and efficiency. In this study, we report the generation of an anti-FMDV Asia1 type mAb. Three mAbs 1B8, 5E1 and 5E2 were screened after immunization of mice with recombinant VP1 protein of FMDV. The specificity, stability, titers and neutralization activity of the mAbs were analyzed.
HTML
-
The YNBS/58 virus strain and O, A and C type FMDV used in this study is a reference strain obtained from the National Foot-and-Mouth Disease Reference Laboratory of Lanzhou Veterinary Institute, Chinese Academy of Agricultural Sciences (LVI, CAAS).
SP2/0 cells were purchased from ATCC (Manasa, VA), and was cultured in RPMI-1640 supplement with 10% fetal calf serum. BHK-21 cells were preserved by LVI and cultured in DMEM supplement with 10% fetal calf serum.
Complete Freund's adjuvant, incomplete Freund's adjuvant, Horseradish peroxidase, 3-(4, 5-Dimethylthiazoyl2-yl)-2, 8-azaguanine, Tetramethylbenzidine and Dimathyl sulfoxide (DMSO) were purchased from Sigma Chemical Co., USA.
-
Escherichia coli BL21 cells containing pProex-VP1 plasmid (2) were induced with IPTG. Then, cells were harvested and resuspended in 30 mL PBS and 1 mmol/L phenylmethylsulfonyl fluoride was added to the slurry and incubated on ice for 30 min. The cells were then sonicated on ice for 40 min and 20% deoxycholic acid was added to a final concentration of 2%. After mixing and incubation at room temperature for 10 min, the lysate was centrifuged at 800 ×g for 30 min at 4℃. The procedures for VP1 protein purification were carried out according to manufacturer 's instructions (His-tag protein purified kit, Invitrogen). Fractions were collected into 1.0 mL tubes and analyzed by SDS-PAGE, the other recombinant proteins were stored in aliquots at -70℃ until use.
-
Female BALB/C mice of 5-6 weeks old were immunized subcutaneously with 20 mg of purified VP1 protein in an equal volume of complete Freund's adjuvant. Three identical boosters emulsified in incomplete Freund's adjuvant were given at 4 week intervals. Mice were boosted with the same antigen in PBS by intraperitoneal injection 3-4 days before fusion. The two immunized mice used for each fusion were sacrificed by overdose anaesthesia. A single-cell splenocyte suspension was obtained for fusion. DMEM with 10% fetal bovine serum was used for fusion and subcloning. Immunized spleen cells were fused with myeloma cells at 5–10: 1 ratio in the presence of 50% polyethylene glycol (Merck). The cells were plated out in semisolid medium (Stem Cell) and incubated at 37℃ in humidified 5% CO2 atmosphere (3). After 2 weeks, single colonies were transferred to 96-well culture plates. Hybridoma supernatants were screened using an indirect ELISA (developed by our Lab). The positive hybridomas were subcloned using the limiting dilution technique. The mAb isotyping was performed using a mouse monoclonal antibody isotyping kit (Isostrip, Roche).
-
The isotype of three mAbs was determined by adding 25 μL of the cell culture supernatant containing 1B8, 5E2 and 5E2, respectively, with 200 μL assay buffer to wells coated with each of the rabbit anti-mouse antibodies from the mouse MonoAb ID kit (Sigma) against IgG1, IgG2a, IgG2b, IgG3, IgA and IgM. Detection of bound mAb was by goat anti-rabbit IgG-HRP conjugated antibody (Sigma). IEF was performed in IEF gel pH 3-7 (Invitrogen). Elec-trophoresis was run following the manufacturer's protocol.
-
A 96-well flat-bottomed plate was coated with serially diluted FMDV Asia1 type cell virus and VP1 protein respectively. Plates were then washed three times with PBS (containing 0.05% Tween 20), and then blocked with 1% BSA at 37℃ for 2 h. Three mAbs were added (100 ng/well) and incubated at 37℃ for 2 h. After washing with Tween/PBS, an HRP-conjugated goat anti-mouse IgG (Sigma) was used for detection.
-
Purified (Saturated ammonium sulfate and Sephacry S-300HR)1B8, 5E1 and 5E2 were subjected to SDS-PAGE according to the standard method described by Laemmli (10) and transferred to 0.45 μm nitrocellulose membranes (Bio-Rad) for 1 h. The blots were then blocked with Tris-buffered saline (TBS) containing 0.1% (w/v) casein for 2 h at room temperature. After washing three times with TBS, the blots were incubated for 1 h at 37℃ with TBS. The membranes were washed three times with TBS and incubated with goat anti-mouse IgG conjugated to HRP (Bio-Rad Laboratories, Hercules, CA) for 1 h at 37℃. Following the washes with TBS, the plates were incubated with the enzyme substrate solution containing 0.5 mg/mL 4-chloro-1-naphthol, 0.15% (v/v) hydrogen peroxide and 25% (v/v) methanol.
-
The procedures of microneutralization (MN) assay for the detection of mAb neutralization activity were followed with certain modifications (5). In general, serial diluted mouse ascitic fluid and equal volume of YNBS/58 virus (100 TCID50) prepared in MEM with 2% FCS were mixed and incubated at 37℃ for 1 h. Each virus/diluted mAb mixture was then applied to eleven wells of a 96-well microtiter plate. BHK-21 cells 5×104 per well were also added. After incubation for 2 days at 37℃, the fifty percent end point of neutralization titers for YNBS/58 were calculated. The amount of virus actually used per well should contain 100 TCID50. Neutralization assays were repeated at least two times.
-
The specificity, stability and titers of mAbs were examined by ELISA (the procedure as described previously). For the specificity, the three mAbs were reacted with O, A and C serotypes of FMDV and SVDV. The stability and titers of prepared mAbs were analysed by ELISA in different passaged generations.
Viruses and cells
Preparation of FMDV recombinant VP1
Mice immunization and generation of hybridoma cells
Isotying and isoelectric focusing (IEF)
ELISA
Western blot analysis
Neutralization assay
mAbs characteristic analysis
-
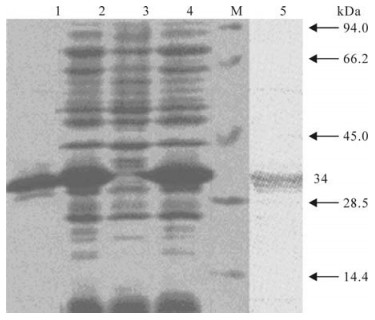
The VP1 protein was purified by one-step affinity purification using glutathione Sepharose 4B as described in Refs (9, 11, 12). The presence of recombinant protein in the eluted fractions was confirmed by SDS–PAGE and Western blot using an anti-GST monoclonal antibody with HRP conjugates. The recombinant VP1 protein was 34 kDa, which accounted for 30 % of total protein in E.coli lysates (Fig. 1).
-
In this study, three mAbs (1B8, 5E1 and 5E2) were obtained. All of them were produced from the mice abdominal cavity. SDS-PAGE showed that the molecular weights of the heavy chain and light chain were about 45.0 and 25.0 kDa (Fig. 2), which was consistent with the predicted molecular weight.
-
In the neutralization assay, the result indicated that prepared mAbs could inhibit YNBS/58 virus from infecting BHK-21 (Table 1). When the mAbs dilution ratio reached to 1:1024, 50% cells still survived; this showed that the titers of mAbs were more than 1: 1024.

Table 1. Detection of mAbs neutralization titers by virus neutralization test
-
In this test, FMDV O, A and C type and swine vesicular disease (SVD) were used to detect the specificity of prepared mAbs. The results indicated that no cross reaction was found with SVD and FMDV O, A and C type antigens (As shown in Table 2).

Table 2. The results of the cross-reaction test
-
In the isotype test, 1B8 was found to be from IgG1 whereas 5E1 and 5E2 belonged to IgG2b. As shown in Table. 3, the ascites titer of mAbs was between 2-5×106. In the stability test, the titers of prepared mAbs were invariably maintained when passaged to thirty generations (as shown in Table 3). All of these results showed that the developed mAbs possessed good specificity and high titers.

Table 3. The ascites titer and stability of the screened monoclonal antibodies
Purification of VP1 protein
mAb production
Neutralization assay
Cross-reaction
Isotype identification
-
Four major procedures: purifying VP1 protein, im-munization BABL/c mouse with purified VP1 protein, fusion spleen cell and SP2/0 cell and screening mAbs, were undertaken in this study. Before fusing to the cell, the antibody titer of the immunized BALB/c mouse showed a titer as high as 1 024, which was a good preparation for this test. In this study, we also found that using expressed protein immunized BALB/c is a good strategy to prepare mAb, since this method would avoid the possibility of live virus escaping from laboratories and other places (6).
Three anti-FMDV Asia1 type mAbs, 1B8, 5E1 and 5E2, were raised and characterized. ELISA and Western blot analysis showed that the prepared mAbs was highly specific to Asia1 type FMDV and did not cross-react with SVD and FMDV O, A and C type antigens. Moreover, prepared mAbs were shown to have an excellent neutralizing activity against Asia1 type FMDV. The neutralizing activity of prepared mAbs was at least 1:1024 (as shown in Table 1, Table 2 and Table 3). Because of this good quality, the prepared mAbs can be used to develop various FMDV type-independent tests for diagnosis and analysis of the antigen epitopes of the FMDV Asia1 type.













 DownLoad:
DownLoad: