-
Human cytomegalovirus (HCMV), a member of the Herpesvirus family, is the most common etiologic agent of congenital anomalies of the central nervous system (CNS) caused by intrauterine infection in humans, with an average incidence of approximately 1.0% among live births (20). It is estimated that 5%-10% of embryos with congenital HCMV infection will have severe neurologic damage at birth, such as microcephaly and perivascular calcification (16). To date, the mechanism concerning the injuries induced by this kind of viral infection remains uncertain.
Nerve growth factor (NGF) is a metabolically active peptide, which was first isolated in nerve tissue. NGF plays a key role in differentiation, development and survival of sympathetic and sensory nerve cells, neural stem cells and cholinergic neurons of the central nervous system (CNS) (2, 12). Apoptosis and cell death may be induced by NGF withdrawal from culture medium of several nerve cells (6, 15, 17). During the embryonic stage, NGF is mainly secreted by neuroglia cells, which are more susceptible to HCMV infection than other nerve cells (2). In this study we analyzed the gene expression of normal and infected human glioma cell lines by RT-PCR and Western Blotting. We found that HCMV infection was associated with alterations in expression of NGF genes.
HTML
-
Human glioma cell line U251 (American Type Culture Collection, Manassas, VA, cell line number: 99522) was purchased from the Shanghai Cell Resource Center of the Chinese Academy of Sciences. U251 cells were propagated in RPMI 1640 (Gibco) medium with 10% fetal bovine serum (FBS, Gibco). Cells were maintained under subconfluent conditions at 37℃ in an atmosphere of humidified 5% (v/v) CO2.
HCMV AD169 (kindly provided by France Pasteur Laboratory and expanded in our laboratory) was titered by plaque titration in human embryonic lung fibroblast (HELF) cells and expressed as the number of plaque-forming units (PFU) per milliliter. The U251 cells were infected by HCMV AD 169 strain with approximately 5 PFU per cell. Viral infections were performed in serum-free medium for 1h at 37℃ and 5% CO2. Virus was removed and replaced with RPMI 1640 containing 10% FBS and cells cultured as described above.
-
6 h post infection, RT-PCR was performed to detect the expression of HCMV IE mRNA. Briefly, 1×106 cells were collected and total RNA was extracted by Trizol (Invitrogen). Reverse transcription was synthesized into cDNA by Oligo (dT), and the total volume was 20 μL containing 2 μL total RNA. Plasmid pRSV72 was diluted by 1:100 as the template for IE amplification PCR and used as the positive control. Two primers for HCMV IE were designed according to the whole gene sequence of HCMV strain AD169 from GenBank (NC_001347). The primer pairs used were: IE forward (5'-CAAGAGAAAGATGGACCC TG-3') and reverse (5'-ACGAGTTCTGCCAGGAC ATC-3'); β-actin forward (5'-ACCGTGGAGAAGAG CTACGA-3') and reverse (5'-GTACTTGCGCTCAG AAGGAG-3'). PCR amplification was performed for 30 cycles at 94℃ for 1 min, 55℃ for 1 min, and 72℃ for 2 min. The expected sizes of PCR products for detecting IE and β-actin are 242 bp and 109 bp, respectively.
-
Total RNA was isolated from infected cells at various times post-infection using Trizol according to the manufacturer's instructions (Invitrogen). Comple-mentary DNA (cDNA) was obtained by reverse trans-cription of 1 μg of total RNA (Thermoscript; Life Technologies) using the oligo (dT)20 primer.
PCR was performed in a 10-μL volume. cDNA was added to the following PCR mixture: 0.5 U Taq polymerase (Eurogentec, Seraing, Belgium), 0.2 mmol/ L dNTPs, 0.5 μmol/L specific primers and 1.5 mmol/ L MgCl2. Negative control was RT without enzyme. PCR reactions were carried out in a Perkin Elmer Thermal Cycler 9600 (Applied Biosystems, Lennik, Belgium). After 1 min of denaturation at 95℃, cycles (25 for β-actin and 32 for NGF) were 10 s at 94℃, 10 s at 60℃ and 30 s at 72℃. Cycling was followed by 10 min of elongation at 72℃.
The NGF primers used were: NGF forward, 5'-CA-CACTGAGGTGCATAGCGT-3', and reverse, 5'-TGATGACCGCTTGCTCCTGT-3'. The sequence of β-actin primers used were as described above. cDNA was equalized for the expression of the housekeeping gene β-actin. PCR products were electrophoresed in 1.5% agarose gel in Tris-borate/EDTA electrophoresis buffer, stained with ethidium bromide, visualized by UV transillumination and analysed by densitometry. Expected sizes of amplification products were 352 bp for NGF and 109 bp for β-actin.
-
Protein extraction from U251 cells was obtained using RIPA Lysis Buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS) and phenylmethylsulfonyl fluoride (PMSF). After 30 min of incubation at 4 ℃, the lysates were heated at 100℃ for 5 min and centrifuged at 12 000×g for 20 min at 4℃. Samples were run on 10% polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride (PVDF) membrane (Millipore), and the blots stained with amido black to ensure that each lane had an equivalent amount of protein. The membrane with transferred proteins was blocked with 5% dry unfit milk in TBS 1× containing 0.1% Tween 20 (TBST) for 1 h at room temperature, and incubated with anti-NGF rabbit polyclonal antibody diluted 1:200 in TBST for 2 h at room temperature. The membrane was incubated with horseradish peroxidaselabeled secondary antibody for 1 h at room temperature. After washing in TBST, the membranes were developed using ECL reagents and protocol according to Amersham. Lysates were also assayed by Western blot for β-actin. Protein bands were quantified using Quantity One software (BioRad). Paired mean comparisons were performed to assess concentration dependent changes in the intensity of native protein bands.
-
All experiments were performed in triplicate, and results are plotted as mean±SD. The statistical signifi-cance between experimental means was determined with Student's t test, and differences of P < 0.05 were considered significant.
Cell culture and virus infection.
Assays for HCMV entry into U251 cells.
NGF RT-PCR.
Protein extraction and western blotting
Data acquisition and analysis
-
1.2% agarose gel electrophoresis showed a 242 bp IE fragment by RT-PCR amplification from infected cells (Fig. 1). RT-PCR detection of HCMV IE in human glioma cell line U251 cells was positive (lane 2) when compared with its positive control (lane 3), demonstrating that the model of HCMV infection was successfully established.
-
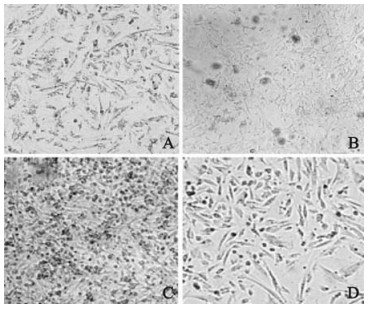
In general, glioma cell morphology appeared normal for the first 3 d after HCMV infection. By 5d, cytopathic effects, such as cellular swelling, cellular confluence and a honeycomb appearance, were evident in more than 50% of cells. After 7 d post infection, these cytopathic effects were observed in almost 100% of cells, with honeycomb-giant cells, and some cell detachment (Fig. 2).
-
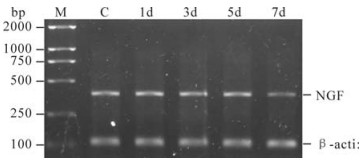
Figure 3 shows β-actin and NGF expression are shown for cells at different time points. The gel shows gene fragments of 110 bp and 350 bp, in accordance with the expected fragment length. There is no detectable difference in expression levels by RT-PCR from the 1st day to the 5th day after infection but by 7 d, expression levels were significantly reduced (0.521±0.053) when compared to day 0 (0.824±0.176) (student's t, P < 0.05). However, the NGF albumin level of the Control Group at different time points does not significantly change.
-
To further verify the results of PCR, western blotting was used to observe the changes of NGF protein expression in U251 cells infected with HCMV. Consistent with the data from RT-PCR, the intensity of bands for NGF decreased at 7d compared with the control (Fig. 4). Significant decreases in the levels of NGF protein were observed using this immunomethod. However, compared with normal cells, the NGF protein remained present in infected cells at 7d when the cells were examined.
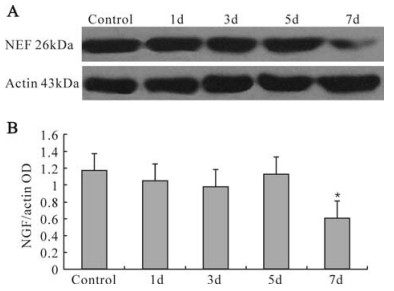
Figure 4. Expression of NGF and β-actin. A: Western blot detection of normal control and infected U251 cells. 1d~7d: 1, 3, 5 and 7 d since U251 cells were infected with HCMV, respectively. B: Statistical analysis of corresponding volume. The 7 d volumes were significantly (*P < 0.05) reduced in infected U251 cells. compared with control group.
Confirmation of HCMV infection model
Morphological changes in cells after viral infection
NGF mRNA expression of U251 Cell
Expression of NGF protein
-
NGF is one of the first discovered neurotrophic factorsand one of the most important bioactivators of the nervous system with extensive biological effects. NGF is mainly distributed in the hippocampus, cerebral cortex, striatum and cerebellum, as well as in neural stem cells in the lateral ventricle and dentate gyrus region of the hippocampus. NSCs cells have self-renewal and multiplex differentiation potential. The development of NSCs includes multiplication, existence, movement and differentiation, and NGF plays an important regulation role in NSC development. During the development of NSCs, many neural progenitor cells will die (1). NGF cannot directly promote the multiplication of neural stem cells, but it can maintain the survival of neural progenitor cells and increase the quantity of neural progenitor cells (4, 21), induce the movement (5, 9) and differentiation (2, 3) of neural stem cells, and maintain the normal functions of neural stem cells. NGF can promote the differentiation, maintain the growth and development, and enhance the injury repair (19) of neurons, but NGF expression and biological effects differ in different developmental periods of the nervous system. According to previous research, the NGF in the central nervous system during the embryonic period is mainly expressed by astrocytes (8, 14), and NGF is mainly synthesized in nerve cells of the mature brain (7); During the embryonic period, NGF can also maintain the survival of neurons, control the survival quantity and promote the differentiation of neurons. In one study, Ignatovich (11) treated an embryonic animal with NGF antibody, and found the number of neurons decreased by 80%, but the treatment with NGF in an adult animal did not cause the reduction of neuron, which indicates that the neuron has much greater independence on NGF during the embryonic period.
HCMV is one of the main causes of congenital infections and fetal deformations, but the exact teratogenic mechanism is not clear (10). As a neurotropic virus, HCMV can infect various nerve cells, with astrocytes being most sensitive (13, 18). This research confirms for the first time that HCMV infection can induce abnormal NGF gene expression of U251 cells in terms of mRNA and albumen levels. According to these results, the NGF gene expression of U251 cell is clearly down-regulated at the later period of HCMV infection. Therefore it is concluded that during the embryonic period, HCMV can probably down-regulate NGF expression, inducing cell apoptosis (16). This in turn reduces the NGF density causing the abnormal development of neural stem cells and neurons, and leads to deformation through infection of astrocytes. However, regulation of NGF expression and other biological effects are influenced by many other factors, and the regulation functions and mechanisms of HCMV on NGF expression in nerve cells requires further research.














 DownLoad:
DownLoad: