-
Reoviruses serve as useful models to study the entry of non-enveloped animal viruses into their host cells. The outer capsid proteins VP5 and VP7 in Grass carp reovirus (GCRV), which belong to the member of Aquareovirus in family Reoviridea (22), may play important roles to allow viral particles to enter host cells (1, 9).
GCRV causes severe acute hemorrhagic disease in fingerling and yearling grass carp, leading to a high mortality of about eighty percent (8). Morphological study showed that virus particles are arranged in two concentric icosahedral capsids. The inner capsid encloses 11 segmented dsRNA genomes that consists of several protein components (VP1-VP3, VP4 and VP6), which play roles in viral transcription and maintaining core frame stabilization. The outer layer is composed of the VP5 and VP7 proteins, which may be involved in virus-cell interactions (3). Genome sequence and 3D structure reconstruction of GCRV indicate that there are strong identities between GCRV and MRV at the genome and structural protein level (3, 15). Further analysis on the VP5 protein of GCRV, which shares about 24% homology with the μ1 protein of MRV (3), suggests that the protein VP5 might possess a similar mechanism of membrane penetration during virus entry into cells.
Recently, the 3D structure of GCRV has been resolved at 9Å resolution and the location of virus capsid proteins was accurately identified. The determination of the localization of the VP5-VP7complex makes it possible to further investigate the virus-host interaction and membrane penetration. In addition, experimental studies indicate that the complete digestion of VP7 and partial cleavage of VP5 lead to an enhanced infectivity, hinting that VP7 and VP5 play important roles in virus entry into cells. While VP7 has been already purified and has been the focus of studies (26), much remains to be understood regarding the VP5 protein. Therefore, in this study, we focused on the expression of VP5 and analysis of its immunogenicity and the results we present here should be helpful for developing vaccines and determination of the viral penetration mechanism.
HTML
-
CIK(Ctenopharyngodon idellus kidney)cells were used for propagation of Grass carp reovirus (GCRV). The cells were grown in MEM (Gibco) supplemented with 10% (v/v) fetal bovine serum and penicillin (100U/mL)-streptomycin (100mg/mL) (Sigma). GCRV873 was isolated from Shaoyang, Hunan province and stored in the author's laboratory (8, 14).
-
GCRV particles were collected from the infected cell culture supernatant by extraction with differential centrifugation to remove cell debris, ultracentrifugation to pellet the virus, and purification on CsCl2 density gradients to isolate uniform particles as described elsewhere (3). Gradient purified viral particle was resuspended in 1×PBS at a concentration of about 1 mg/mL. The purified virus was then used as antigen to generate the anti-GCRV serum and the remaining sample was stored at -20℃ until further use (10).
-
RNA extraction and cDNA synthesis were performed according to the manufacturer's instruction (Bio Flux, Japan; Toyobo, Japan). The primer 5'-GACTCGAGTCGACACTTCGCACTCTCTC-3' (sense, the XhoⅠ site is underlined) and 5'-CACTGCAGTTCACGCGGGCATGGAAG-3' (anti-sense, the Pst Ⅰ site is underlined) were designed to amplify the GCRV s6 gene based on GenBank sequence (AF403392). The PCR cycle parameters were an initial denaturation at 94℃ for 5 min, followed by 35 cycles consisting of 94℃ denaturation for 1min, annealing at 55℃ for 1min, and 72℃ extension for 2 min and 10 sec. Strand synthesis was completed at 72℃ extension for 10 min. The amplified fragment was purified by DNA gel extraction kit (V-gene, Hangzhou, China).
-
The purified PCR product was cloned into pRSET-A (Invitrogen, Carlsbad, USA) vector to construct prokaryotic expression plasmid pR/GCRV-VP5. After digestion by XhoⅠ and Pst Ⅰ, the product was ligated by T4 DNA ligase and transformed into both DH5α and BL21 (DE3) PLysS (Invitrogen, Carlsbad, USA), and recombinant plasmids were obtained. The resultant positive plasmids were identified by both restriction enzyme digestion and PCR amplification (7), and then sequenced by Invitrogen Biotechnology Inc (Shanghai, China) to further confirm the inserted gene.
-
The positive colonies transformed into BL21 (DE3) PLysS cells were grown overnight at 37℃ in SOB medium, inoculated into fresh medium and grown for an additional 3h and then induced by IPTG at a final concentration of 1mmol/L at 37℃ for time course expression. The collected bacteria were lysed, and both supernatant and sediment of cell lysate were denatured by boiling for 5 minutes with 2×SDS-PAGE loading buffer. Then the samples were subjected to SDS-PAGE gel (10%) for electrophoresing as described elsewhere (10).
-
The purified VP5 protein and gradient purified GCRV sample were each used as antigen to raise antiserum in New Zealand white rabbit. The rabbit was firstly immunized with 400 μg purified protein that was emulsified in an equal volume of Freunds complete adjuvant (FCA). The inoculations were performed by intramuscular injection. Freunds in-complete adjuvant and 400 μg purified protein were used in booster injection two weeks later. Three days after the last immunization, the rabbit was sacrificed to prepare the antiserum.
-
To purify the recombinant protein, a large scale volume of VP5 protein was induced for 4 h at 37℃ in BL21 (DE3) PLysS cells. Since there is a His-tag region in the N-terminal of the recombinant proteins, MagExtractor-His-tag purification kit (Toyobo, Japan) was used to purify the interest fusion protein. The kit has a nickel-charged bead that can be used for affinity purification of fusion proteins containing the 6xHis tag. Proteins bound to the bead may be eluted with either low pH buffer or by competition with imidazole or histidine. Both native and denature conditions were tried for comparative purposes.
The recombinant protein was electrophoresed on the SDS-PAGE, and then semi-dry electrically trans-ferred to the PVDF membranes. After blocking out by 3% BSA for 1h at 37℃, the membrane was incubated at 37℃ for 1h with a 1:100 dilution of the anti-GCRV serum. Then it was incubated with a 1:3 000 dilution of the goat anti-rabbit IgG at 37℃ for another 1h. Finally the membrane was observed by developing with AP substrate solution (NBT/BCIP) for 20 min.
-
The immunogenicity of the expressed VP5 protein was tested by ELISA (6). First, purified GCRV virus with 0.5mg/mL concentration was coated with Na2CO3-NaHCO3 buffer (pH9.6) in a 96-well plate overnight. The coated plate was washed 3 times with wash buffer PBST (1×PBS+Tween20) the next day, and then each well was incubated with a series of dilutions of the anit-VP5 serum. After another washing with PBST, a 1:3000 dilution of goat anti-rabbit alkaline phosphatase labeled secondary antibody was added. Finally, the color was developed with NBT/ BCIP and the OD was read at 405nm. The 1×PBS and normal rabbit serum were used as negative controls.
Cells and Virus
Purification of GCRV virions
RNA extraction and RT-PCR
Plasmid construction
Expression of the recombinant protein
Preparation of antiserum
Purification and analysis of the expressed protein
ELISA assays
-
The cDNA obtained from the RT reaction was used as template to amplify the s6 gene which encoded the outer capsid protein VP5. As shown in Fig. 1.A, the amplified PCR fragment was about 2.0 kb, which matched well with its predicted size.
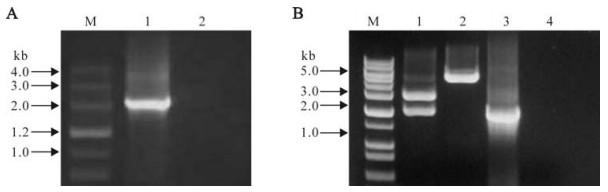
Figure 1. RT-PCR amplification and identification of recombinant plasmid. A: RT-PCR amplification. M, DNA marker Ⅲ; 1, Positive amplification (~ 2.0kb); 2, Negative control by using ddH2O as template. B: Identification of recombinant plasmid. M, 1 kb plus DNA ladder marker; 1, Double enzyme digested recombinant plasmid with XhoⅠ and Pst Ⅰ; 2, Single enzyme digested with Pst Ⅰ; 3, Positive amplification of s6; 4, Negative Control amplification of s6.
After the s6 gene was cloned with the prokaryotic expression vector pRSET-A to construct the recombinant plasmid, it was confirmed by restriction enzyme digestion and PCR amplification. The result of the double enzyme digestion showed that it was very similar to the predicted size which was the combination of the s6 product and the plasmid vector of pRSET-A, which were 2.0kb and 2.9kb, respectively (Fig. 1.B). The validity was further confirmed by sequence analysis (data not shown).
-
The prokaryotic expression plasmid pR/GCRV-VP5 that was transformed into BL21(DE3) pLysS was induced by IPTG for 1, 3, 5 h at 37℃, respectively. Using pRSET-A transformed BL21(DE3) pLysS as control, SDS-PAGE showed that there was an over-expressed band with the pR/GCRV-VP5 transformed BL21 (DE3) pLysS, which was consistent with the predicted molecular weight of ~70kDa (fig. 2A). We found that the over-expressed VP5 protein accumulated in the pellets as inclusion bodies rather than in the supernatant. For the detailed verification of the expressed protein, we induced the protein by IPTG for 0, 1, 2, 3, 4, 5 h at 37℃, respectively. The results, shown in Fig. 2B, further indicated that the recombinant protein was over-expressed, and reached a peak at 3 h post IPTG induction.
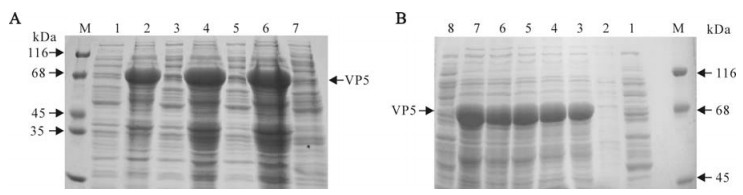
Figure 2. Time course expression of pR/GCRV-VP5. A: M, Standard protein marker; Lane 1, pRSET-A negative control induced by IPTG; Lane 2/4/6, pR/GCRV-VP5 pellets induced by IPTG for 1, 3 and 5 h, respectively; Lane 1/3/5, pR/GCRV-VP5 supernatant induced by IPTG for 1, 3 and 5 h, respectively. B: M, Standard protein marker; Lane 1, pR/GCRV-VP5 supernatant induced 3 h by IPTG; Lane 2-Lane 7, pR/GCRV-VP5 pellets induced by IPTG for 0, 1, 2, 3, 4 and 5 h, respectively; 8, pRSET-A negative control induced by IPTG.
-
Because of the His-tag region in the N-terminal of the recombinant proteins, we chose his-tag Mag-Extractor purification kit. The beads, which were chelated with nickel, had a high ability to combine with his-tag selectively. Compared with the native conditions, we found the denaturing conditions had a much better yield of purified protein, (Fig. 3A).
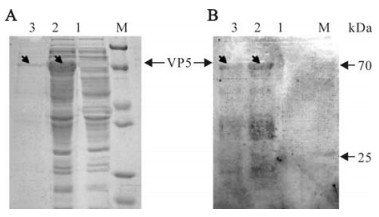
Figure 3. SDS-PAGE analysis and Western blot of expressed VP5 protein. M, Standard protein marker. A: Coomassie brilliant blue stained SDS-PAGE gel. Lane 1, cell lysate of pRSET-A empty vector transformant induced by IPTG at 3h as negative control; 2, pR/GCRV-VP5 cell pellets induced by IPTG at 3 h; 3, the purified VP5 protein. B: Western blot analysis corresponding to the protein gel in A with anti-GCRV antibody.
To test the specificity of the antiserum, the expressed and purified protein was immunoblotted with anti-GCRV serum. It appeared the IPTG induced pR/GCRV-VP5 cell pellet and purified protein were able to bind immunologically to anti-GCRV serum; no visible band could be detected with the expressed cell lysate of pRSET-A empty vector transformant, demonstrating that the expressed VP5 protein is specific to anti-GCRV serum.
-
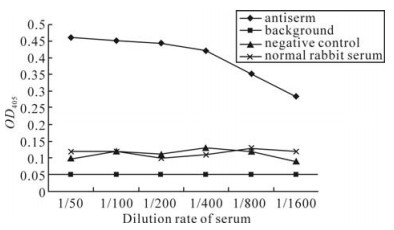
Purified VP5 protein was immunized in New Zealand white rabbit. After twice enhanced immunization, the primary titer of antiserum was tested. To further verify the antigenicity of expressed VP5 and its antibody specificity, the titer of anti-VP5 serum was then determined with antigen coated GCRV particles by ELISA detection. It was found that the minimum OD405 value of polyclonal anti-VP5 serum at a dilution of 1:800 was 0.35, which is about 3 times higher than the value for normal rabbit serum. (Fig. 4), indicating the anti-VP5 serum is specific to GCRV and the expressed VP5 protein has a good immunogenicity.
RT-PCR amplification and plasmid construction
Time course expression of recombinant plasmid
Purification and analysis of the expressed protein VP5
Immunogenic analysis of the expressed protein
-
Among the seven structural proteins of GCRV, VP7 and VP5 are the outer capsid proteins of the virus, which correspond to the σ3 and μ1 proteins of MRV respectively in both protein homology and particle location (3, 9, 10). Significantly, the identity of the N-terminal region up to the autolytic cleavage site (Asn42-Pro43) with the corresponding region in μ1, suggests that VP5 may program a similar membrane penetration mechanism during virus entry into host cells (3, 25).
Previous reports have proposed a model for reovirus entry which is related to the mechanism for μ1. Once the virus has attached to membrane of the host cell, a conformational change of μ1 is triggered. Then, after μ1 undergoes a special (distinct) auto-cleavage, a myristoyl group, which is originally packed inside the protein, is released to the surface and then inserted into cell membrane; by this mechanism the virus is able to enter into the cell (12). With respect to the autolytic cleavage site (Asn42-Pro43), the N-terminal region of VP5 in GCRV is highly conserved with μ1, suggesting that a similar myristoyl switch mechanism may be employed by VP5 for membrane penetration as μ1 (12).
GCRV, the first identified agent from a outbreak of hemorrhagic disease affecting a vast majority (85%) of fingerling and yearling grass carp from southern China, is considered to be the most pathogenic aquareovirus (3). Due to its virulence, it is necessary to study the infection and pathogenesis mechanism of the virus. In this report, we have successfully expressed VP5, a full length outer capsid protein of GCRV, in prokaryotic cells, which is immunologically related to the epitope of GCRV. It should be mentioned that the recom-binant protein VP5 was overexpressed, and there were no degraded protein products found in both cell lysate and purified protein samples in our study, which is distinct from an earlier brief report on expression of the GCRV M6 gene (20). The over-expression of VP5 protein and the determination of its high immunogenicity will give an opportunity for further studies on understanding the mechanisms of virus-cell interaction as well as developing an effective vaccine for immunization.













 DownLoad:
DownLoad: