-
DNA vaccines represent a promising technology that uses DNA to encode the antigen(s) of interest, instead of inoculating with attenuated or inactivated microbes or isolated antigens. The cells containing DNA vaccines express the antigen and present it to the recipient's immune system. DNA vaccines have many potential advantages. No unsafe unfectious agents are involved and they can induce both humoral and cytotoxic T cell responses, even without replication. There is a potential for encoding multiple immunogenic epitopes with the purpose of raising protection against several diseases by a single vaccine. DNA vaccines may be manufactured quickly and easily, and they are relatively stable. Therefore, for clinical trials, the U.S. FDA (Food and Drug Administration) has provided manufacturers with guidance on toxicology studies. Among these recommended studies are repeated-dose toxicology, biodistribution, and integration analyses.
A Hantaan virus DNA vaccine has been tested in vivo for immunogenicity, and got a satisfied result [5, 6, 11]. Thus, it provides a very good perspective for preventing Hemorrhagic fever with renal syndrome (HFRS). HFRS, caused by Hantaan virus (family Bunyaviridae, genus Hantavirus), is a rodent-borne zoonosis widely distributed over China, particularly in the northeast part of China. It is characterized by a period of fever, hemorrhagic manifestations and renal impairment. As of now, there is no effective treatment.
In this study, we constructed a Hantaan virus DNA vaccine containing an S antigen gene fragment coding Hantavirus strain 76-118 nucleocapsid protein (NP) and observed the kinetics of the fusion protein generated following intramuscular delivery of pEGFP/S in mice and examined the persistence of the plasmid in several organs (liver, lung, kidney, spleen, brain and local muscle) we hope the study will conduce to the research of DNA vaccine pharmacokinetics and HFRS pathogenic mechanism[9].
HTML
-
A DNA fragment encoding the S segment fragment was digested from the plasmid pTargetS (provided by Prof. Luo, China Medical University, China) by using EcoR Ⅰ and Sal Ⅰ. The EcoR Ⅰ-Sal Ⅰ fragment was ligated into EcoRⅠ-SalⅠ digested eukaryotic expression vector pEGFP-C1 to generate pEGFP/S. We constructed the recombinant plasmid pEGFP/S by inserting the S fragment into pEGFP-C1, which encodes the S gene in Hantavirus.
-
All plasmids were purified with the Qiagen Plasmid Midi Kits (Qiagen, Germany). For each transfection experiment, Vero-E6 cells, that were purchased from Shanghai Life Science Academy, Chinese Academy of Sciences, were seeded in 24-well plates and grown for about 24 h until they were above 90% confluence, then transfected with an equal amount of the reporter plasmid using Lipofectamine 2000 (Invitrogen, USA), according to the manufacturer's instructions. Five hours later, serum-free DNA-containing medium was replaced by fresh growth medium and the cells were harvested 48 hours after transfection. The slides were incubated with anti-NP IgG monoclone antibody (provided by Prof. Luo, China Medical University, China) at 37℃ for 30 min and kept at 4℃ overnight. They were then incubated with goat anti-rat IgG antibody (Invitrogen, USA) and viewed under a fluorescence microscope.
-
Six-week-old female BALB/c mice (purchased from Animal Research Institute, China Science Academy) were bred and maintained in the Medical Laboratory Animal Center, China Medical University. The mice were immunized with the plasmids, containing the S fragment. Control animals included mice injected with the PBS and the empty vector pEGFP-C1. Each group contained 10 animals. The dose of 100μg of plasmid DNA in 100μL was delivered intramuscularly to each mouse in the right tibialis anterior muscle. One week before the DNA immunization, 100μL of 0.5% bupivacaine hydrochloride (Sigma, Germany) in isotonic NaCl were administered intramuscularly into the site of DNA inoculation.120 six-week-old female BALB/c mice were purchased from the Animal Research Institute, Chinese Academy of Sciences.
-
On days 3, 7, 15, 30 and 60 after injection, each two mice in experimental group and in two control groups were sacrificed and random sections were taken from the liver, spleen, kidney, lung, brain and local muscle tissue and 7μm thick frozen serial sections were prepared immediately, then observed under a fluorescence microscope.
-
GFP-NP protein was extracted from the isolated liver, spleen, kidney, lung, brain and local muscle tissue on days 3, 7, 15, 30 and 60 by using a Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, Haimen, China) according to the protocol provided by the manufacturer. Protein concentrations were determined using the BCA Protein Assay Kit according to the protocol provided by the manufacturer (Beyotime Biotechnology, Haimen, China). 100μl of supernatant was added to an equal volume of 2×SDS sample buffer and boiled for 5min at 100℃. The samples were then stored at -80℃ until analyzed. The electrophoretic mobility of the proteins analyzed in this study was determined by SDS polyacrylamide gel electrophoresis using 15% acrylamide concentrations. After electrophoresis, the proteins were transferred electrophoretically to a nitrocellulose filter membrane that was then blocked for 4h in a solution of 8% nonfat dry milk in Tris-buffered saline containing 0.1% tween (pH7.6) at RT. The membrane was then incubated overnight at 4℃ with anti-NP IgG monoclone antibody prior to the Western blotting. Bands were washed four times, after which they were incubated with goat anti-rat IgG antibody for 2h and again washed four times. The blots were developed using an ECL Western blotting kit as recommended by the manufacturer. In this test, β-catenin as a control.
Construction of DNA vaccines
Fusion protein expression in cultured cells
Immunization of mice
Treatment of sample
Western blotting
-
The viability of the prepared plasmids were verified restriction digestion and by DNA sequencing (Fig. 1).
-
Many cells exhibiting a fluorescent green were found in the cytoplasm of pEGFP/S-transfected Vero-E6 cells, and the cells became a fluorescent red when incubated with both polyclonal anti-NP IgG (Invitrogen, USA) and goat anti-mouse IgG antibody (Invitrogen, USA). This indicates that the plasmid pEGFP/S has the ability to express both nucleocapsid protein and GFP in eukaryotic cells (Fig. 2).
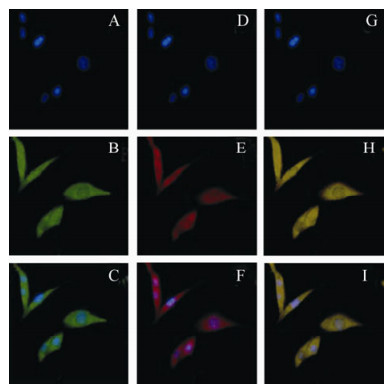
Figure 2. Recombinant fusion protein could be expressed in eukaryotic cells. A-C, Recombinant fusion protein detected in the cytoplasm of Vero cells transfected with pEGFP/S under a fluorescence microscope. Nucleus detected by DAPI; D-F, Recombinant fusion protein detected by polyclonal anti-NP IgG and goat anti-mouse IgG antibody in the cytoplasm of Vero cells transfected with pEGFP/S;G=A+D; H=B+E; I=C+F.
-
The liver, lung, kidney, spleen, brain and local muscle of all each two mice in experimental group were detected obvious green fluorescence on days 3, 7, 15, 30 and 60 after immunization (Fig. 3).
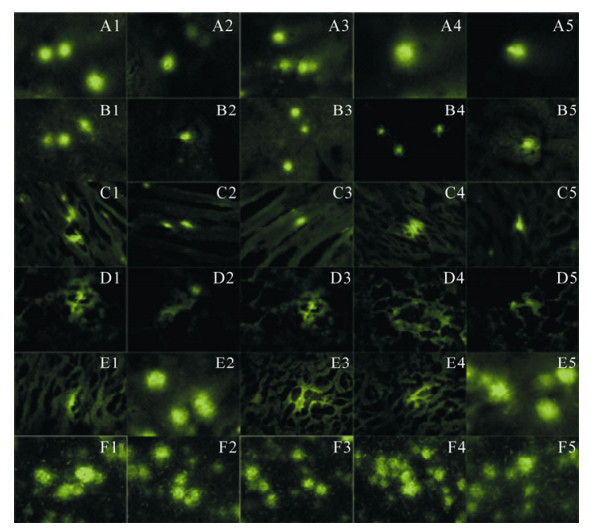
Figure 3. GFP-NP detected green fluorescence in several mice organs at follow days after inoculation under fluorescence microscope. A1-A5, GPF-NP expressed in mice brain on days 3, 7, 15, 30 and 60; B1-B5, GPF-NP expressed in mice liver on days 3, 7, 15, 30 and 60; C1-C5, GPF-NP expressed in mice local muscle on days 3, 7, 15, 30 and 60; D1-D5, GPF-NP expressed in mice pulmonary on days 3, 7, 15, 30 and 60; E1-E5, GPF-NP expressed in mice renal on days 3, 7, 15, 30 and 60; F1-F5. GPF-NP expressed in mice spleen on days 3, 7, 15, 30 and 60.
-
We detected the GFP-NP protein in the isolated liver, lung, kidney, spleen, brain and local muscle on days 3, 7, 15, 30 and 60 by using Western blotting (Fig. 4), and found that the titers of the antibody on days 3, 7, 15, 30 and 60 in these organs have a similar rule.
Identification of DNA vaccines
Expression of Recombinant Fusion Protein in vitro
Expression of the Hantaan Virus DNA Vaccine in Important organ
Western blotting
-
There has been much progress since the initial discovery of the spontaneous transfection of myocytes after intramuscular delivery of plasmid DNA, [12]. Intramuscular administration by simple injection of DNA isn't considered to be an effective route of DNA vaccination, though it has some unique advantages, such as large capacity, safety and convenient, et al. Furthermore, most researchers believe that the skeletal muscle system, including cardiac and skeletal muscle, is the most effective system uptake exogenous DNA. It has been reported that the capacity of muscle cells to uptake exogenous DNA is 100~1, 000 times stronger than cells in other organisms [4]. Though the exact mechanism by which plasmid vaccine antigen presentation in muscle remains unclear [1~3], the antigen presenting cell (APC), particularly dendritic cells (DCs), have been considered to play an important role in antigen presentation[10]. DCs as the most important APC root in negative predecessor MHC Class I cells that both monocyte and granulocyte possess. Because DCs can enter all the organs of the body except for the brain, we consider that an injected plasmid should be widely distributed and expressed throughout the body. Our results indicate that the plasmid has indeed been distributed in such a manner after intramuscular injection.
Immunologic memory, as the foundation of the practice of vaccination[8], is the most important factor determining the ability of the immune system during the secondary response. In DNA immunization, the immunologic memory cells are generated after inoculation during the first response, when expression levels of the target protein are the highest[7]. And immunologic memory may be maintained by the long-term persistence of a DNA vaccine that expresses the target genes continually. Some studies have described this persistent expression phenomenon as a 'depot'[13]. Our study showed that the plasmid has persistent expression that lasts for more than 60 days in vivo after intramuscular injection. Thus, we could regard the organs as a 'depot' for a Hantaan virus DNA vaccine.
In conclusion, we have constructed a fusion DNA vaccine pEGFP/S that expressed the S segment and EGFP and demonstrated that pEGFP/S could express recombinant NP-GFP protein in eukaryotic cells and induce distribution widely and persistent expressed in vivo. Furthermore, a plasmid 'depot' in the organs acts as a booster immunization to maintain the immune responses.













 DownLoad:
DownLoad: