-
Hepatitis C virus (HCV) infection is a blood-borne disease (Lauer G M, 2001), which affects almost 3% of the world's population (Lang K A, 2008) with a morbidity and mortality that are second only to human immunodeficiency virus (HIV) among emerging infections (Lauer G M, 2001). An acute HCV infection may result in chronic liver disease, cirrhosis, hepatic failure, hepatocellular carcinoma (Bartenschlager R, 1993), or death (Koff R S, 2003). Despite the high prevalence of HCV infection (Lanford R E, 2004), the only effective antiviral therapy currently available is interferon (IFN) and ribavirin (Semmo N, 2005). Thus, there is an urgent need for the development of novel immune therapy strategies to combat HCV (Lanford R E, 2004; Weiner A J, 2001). DNA vaccines are an effective approach to induce antigen-specific immunity (Guermonprez P, 2002; Gurunathan S, 2000; Ha S J, 2003; Yang S H, 2006); they are relatively safe, cost efficient, stable, easily produced, and are able to sustain reasonable intracellular levels of antigen expression (Guermonprez P, 2002; Gurunathan S, 2000). DNA immunization can induce cell-mediated immune responses against structural and nonstructural (NS) HCV genes in mice (Bocher W O, 2001; Tokushige K, 1996).
The HCV NS3 gene has been considered as a possible vaccine target (Bartenschlager R, 1993; Pang P S, 2002). NS3 is a ~69-kDa hydrophobic protein that contains a serine protease in its N-terminal region (Hijikata M, 1993) and an RNA helicase in the C-terminal region (Frick D N, 2007). The gene product is essential for viral replication and can suppress the antiviral immune system (Foy E, 2004).
The correlation of NS3-specific T cell responses (Ahlen G, 2005; Jiao X, 2004; Martin T, 1999) with infection clearance makes the HCV NS3 gene an attractive target for DNA vaccines (Nichols W, 1995; Martin T, 1999). Different approaches have been used to enhance DNA vaccine potency (Geissler M, 1997; Lang K A, 2008). Co-administration of cytokines can enhance and modulate the immune response in the desired direction (Geissler M, 1997; Kim J J, 1997; Xiang Z, 1995). IL-12 is a heterodimeric cytokine that plays a key role in cell-mediated immune responses, including activated T cell proliferation and differentiation of CD8+ T cells (Kieper W C, 2002). Therefore, we induced immune responses in a mouse model by co-administration of an HCV NS3 DNA vaccine and IL-12 adjuvant.
HTML
-
The DH5α strain of Escherichia coli competent cells was used as a bacterial host during the transformation. E. coli DH5α cells were grown at 37 ℃ in Luria Bertani (LB) medium, supplemented with 50 μg/mL ampicillin. Chinese Hamster Ovary (CHO) cells were cultured in Roswell Park Memorial Institute medium (RPMI) 1640 (Gibco BRL, Paisley, UK) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine (Sigma-Aldrich, Saint-Louis, Missouri, USA), at 70% confluency 20 h before transfection.
-
We constructed a pcDNA3.1-HCV NS3 plasmid containing a HCV NS3 expression cassette under human cytomegalovirus (HCMV) immediate-early promoter. The HCV NS3 gene was isolated using sera from HCV-infected patients (genotype 1a). Purified polymerase chain reaction (PCR) products were cloned into a pTZ57R/T cloning vector and were confirmed by sequencing. The digested gene was subcloned into a pcDNA3.1 eukaryotic expression vector (Invitrogen, Canada). E. coli DH5α strain bacteria were transformed with the plasmids and plated on LB plates containing 100 µg/mL ampicillin. The selected colonies were extracted using a Qiagen extraction kit (Qiagen, Hiden, Germany) according to the manufacturer's instructions. Plasmid pcDNA3.1-IL12 was kindly provided by Dr T. Sakai (University of Tokushima).
-
The transient expression of pcDNA3.1-HCV NS3 was confirmed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis. CHO cells at 70% confluency were seeded into a 6-well micro-plate and incubated overnight in complete medium without antibiotics and transfected with the recombinant plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
The proteins in the lysate were diluted in SDS, loaded onto polyacrylamide/SDS gels, isolated by electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) western blotting membrane (Roche, Germany) by electrophoresis for 1 h at 90 V. Membranes were blocked in 3% bovine serum albumin (BSA) in phosphatebuffered saline (PBS) for 1 h. NS3 protein was detected using a monoclonal anti-HCV NS3 antibody (Abcam, Cambridge, UK). The specific band was visualized with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G antibody (Sigma). Color was developed by incubating the membrane in alkaline phosphate buffer containing tetramethylbenzidine substrate solution.
-
Female C57BL/6 mice (5–8 weeks old) were purchased from Institute Pasteur of Iran. The mice were acclimated for 1 week before the experiment. The mice were divided into five groups of seven mice.
The first group was immunized intramuscularly with 90 µg both of HCV NS3 and IL-12 DNA vaccines. The second group of mice was injected with HCV NS3 DNA vaccine. The third group received IL-12 DNA vaccine alone, and the fourth were given a combination of IL-12 and pcDNA3.1 plasmids. The fifth group received pcDNA3.1 as a negative control. To evaluate HCV DNA vaccine efficiency, mice were immunized by intramuscular injection into the quadriceps with 90 µg of each DNA vaccine. Immunizations were administered three times on days 0, 14, and 28. The mice were sacrificed 2 weeks after the third immunization. All experiments were in accordance with the Animal Care and Use Protocol of Golestan University of Medical Sciences.
-
Two weeks after the final immunization, single-cell suspensions of mononuclear cells obtained from immunized mice were used for lymphocyte proliferation assays. Erythrocytes were lysed at room temperature using lysis buffer (NH4Cl, KHCO3, and Na2EDTA). The cells were washed and resuspended in RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin. The density of cells in suspension was counted with a heomocytometer under light microscopy, and viability test was carried out using the Trypan blue dye exclusion method. The splenocytes were cultured at a concentration of 2 × 105 cells/well in 96-well plates in the presence of 1 µg/mL NS3 antigen, 5 µg/mL phytohemagglutinin (PHA) (Sigma), or media. After 48 hours of incubation, 10 μg/mL of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (Sigma) was added to each well and incubated for 4 h at 37 ℃ in 5% CO2. Dimethyl sulfoxide (DMSO, 100 µL) was also added to dissolve the formazan crystals. Then, the plates were read at 540 nm, and the results were expressed as a stimulation index (SI) that was determined as follows: optical density (OD) values of stimulated cells (Cs) − relative cell numbers of unstimulated cells (Cu)/relative OD values of unstimulated cells (SI = (Cs – Cu)/Cu).
All tests were performed in triplicate for each mouse (Ghaemi A, 2007).
-
Splenocyte CTL activity was determined by lactate dehydrogenase (LDH) release assay. Two weeks after the last immunization, single-cell suspensions of splenocytes were prepared and used as effector cells. A precise number of 4 × 104 EL4 cells in a volume of 100 μL were incubated with effector cells (100 μL) at different effector/target ratios. For preparation of the target cells, EL4 cells were stimulated with 1 µg/mL HCV NS3 antigen.
LDH release due to cell lysis was measured with an LDH release assaying kit (Takara) according to the manufacturer's instructions. For low and high control wells (spontaneous release and maximum release, respectively), 100 μL of assay medium or 2% Triton X-100 was added instead of the effector cell suspension.
The percentage of specific cytolysis was determined by the following formula:
Specific cytolysis (%) = (OD] of experimental LDH release − OD of spontaneous LDH release of effector cells − OD of spontaneous LDH release from target cells)/ (maximum LDH release of target cells − OD of spontaneous LDH of target cells) 100%. All determinations were performed in triplicate (Ghaemi A, 2010).
-
Two weeks after the third immunization, mononuclear cells from spleens of immunized mice at a concentration of 2 × 106 cells/well in 24-well plates (Nunc, Denmark) were incubated for 2 days in a total volume of 1.5 mL RPMI 1640 supplemented with 10% fetal calf serum (FCS), 1% L-glutamine, 1% hydroxyethyl piperazineethanesulfonic acid (HEPES) as a buffering agent, 0.1% 2ME, 0.1% penicillin/streptomycin and pulsed with NS3 antigen at 37 ℃ in 5% CO2. The cell supernatants were collected and assayed for the presence of IFN-γ and IL-4 using commercially available sandwich-based enzyme-linked immunosorbent assay (ELISA) kits (in comparison to unstimulated controls) (R & D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. All tests were performed in triplicate for each mouse (Ghaemi A, 2009).
-
One-way analysis of variance (ANOVA) was used to compare results among the five different groups. The statistical software SPSS version 18.0 was utilized for statistical analyses (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when p < 0.05.
Bacterial strain and cell line
DNA vaccine construction
Transfection and expression analysis
Vaccination
Lymphocyte proliferation assay
Cytotoxic T Lymphocytes (CTL) assay
Cytokine secretion assay
Statistical analysis
-
HCV NS3 gene expression was confirmed in transfected CHO cells using western blot analysis with a mouse anti-hepatitis C NS3 antibody (Abcam). Non-transfected CHO cell lysate was used as a negative control. The transfected CHO lysate showed a single band at about 63 KDa for NS3 (Supplementary Fig. 1B).
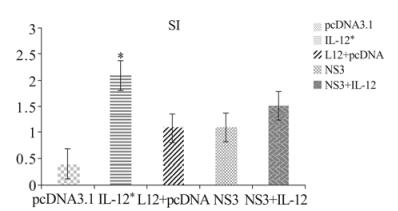
Figure 1. Cell proliferation and SI in immunized mice. C57BL/6 mice three times immunized with pcDNA3.1, IL-12, IL-12+ pcDNA3.1, NS3, NS3+ IL-12. Two weeks after last injections, spleen cell in 5 groups were removed and lymphocyte proliferation measured by MTT assay. Formazan crystal formation after incubation with MTT reagent was determined by solving the crystals in MTT detergent, and the OD was read at 540 nm. NS3+IL-12, IL-12 groups demonstrated higher proliferation and SI than in other groups. * p < 0.05 comparing to other groups.
The presence of plasmids in E. coli DH5α cells was confirmed by growth of single colonies on LB agar plates containing ampicillin, and plasmid extraction was carried out in maxi-preparations. The purity and identity of the pcDNA3.1 plasmid was confirmed by 1% agarose gel electrophoresis (Supplementary Fig. 1A).
-
Splenocytes from all five groups were tested with a lymphocyte proliferation assay. As shown in Fig. 1, the NS3 + IL-12 (1.52 ± 0.27) and IL-12 groups (2.12 ± 0.06) had a much better proliferation response than the NS3 and negative groups. The results also revealed a significant difference in the lymphocyte proliferation index in the IL-12 group compared with the NS3 + IL-12 group (p < 0.05) (Fig. 1, OD values in supplementary table 1).
-
We examined LDH release in the five immunized groups with an LDH assay in 96-well plates. As shown in Fig. 2, lymphocytes in mice vaccinated with HCV NS3 (84.43 ± 6.65) and NS3 + IL-12 (84.44 ± 8.67) had the highest LDH release and CTL activities compared with other groups. The IL-12 group (73.17% ± 10.91) had significantly increased specific CTL activity compared to the negative control group. The results also revealed statistically significant differences among the NS3 + IL-12, NS3, and IL-12 control groups (p < 0.05), was not found (OD values in Supplementary Table 2).
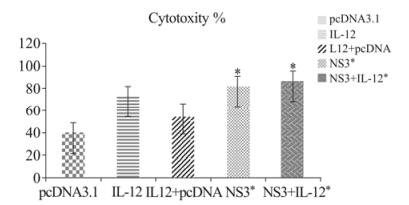
Figure 2. Cytolytic assay to explain LDH release in mice vaccinated with plasmid pcDNA3.1, IL-12, pcDNA3.1+IL-12, HCV-NS3 gene, NS3+IL-12 DNA vaccine with the quantitative measurement of CTL assay. Following 3 times immunization spleens were harvested as described in the Materials and Methods. Data of released LDH were collected and expressed as Mean percent cytotoxicity ± SD. * p < 0.05 comparing to other groups.
-
To determine whether vaccination with the recombinant DNA vaccine could upregulate cytokine secretion and increase specific immune responses, we measured IL-4 and IFN-γ levels in supernatants of mononuclear cells from vaccinated mice that were restimulated in vitro with NS3 antigen. As shown in Fig. 3, splenocytes taken from immunized mice that received NS3 + IL-12 and NS3 vaccines produced higher levels of IFN-γ in comparison to the other groups. Immunized mice that received NS3 and NS3 + IL-12 DNA vaccines had higher levels of IL-4 in comparison to the other groups. This result indicates that the NS3 regimen predominantly produces a mixed Th1 and Th2 response profile.
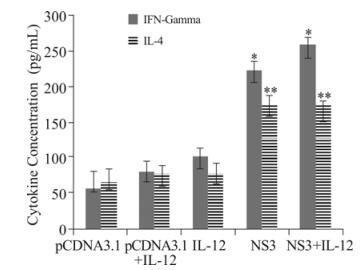
Figure 3. Production of Th1 and Th2 cytokines. Collected supernatants were screened for the presence of IFN-γ and IL-4 to determine the phenotype (Th1 vs. Th2) of the immune responses. The concentration of the cytokines was determined by comparison to a standard curve of serially diluted positive control samples. Bars: means. Whiskers: SD. Each sample was examined in triplicate, and the results are representative of 2 experiments. *, ** p < 0.05 comparing to other groups.
HCV NS3 DNA vaccine construction
Lymphocyte proliferation response
Cytotoxic T Lymphocytes (CTL) assay
IFN-γ and IL-4 secretion levels
-
Currently, there is no prophylactic or therapeutic vaccine for HCV (Lanford R E, 2004) Heterogeneity and high mutagenicity in the HCV genome may be a major obstacle for vaccine design (Pawlotsky J M, 2009; Weck K, 2005). Previous findings indicate that cellular immune responses may be necessary for an effective vaccine (Ghaemi A, 2009). Promising vaccine candidates, such as DNA vaccines, have been shown to have many advantages (Feltquate D M, 1997). The NS3 gene is relatively conserved, making it an attractive candidate for T cell based vaccines (Ahlen G, 2007).
The NS3 gene is important in HCV clearance by inducing specific T cell responses (Andre S, 1998). Previous studies have demonstrated that CD8+ T cells could effectively eliminate NS3/4A-expressing hepatocytes and tumor cells in a mouse model (Ahlen G, 2005; Frelin L, 2004).
With the aim to develop an effective DNA HCV vaccine, we designed and administered an HCV NS3 DNA vaccine and then performed cellular immunity, LDH release, and cell proliferation assays. The results showed that the HCV NS3 gene alone produces moderate immunity; therefore, we administered an IL-12 gene along with the HCV NS3 gene to activate CTL, which plays a critical role in cellular immune responses and lymphocyte proliferation induction.
Our results demonstrated that immunization with the HCV NS3 + IL-12 DNA vaccine resulted in the highest LDH release and T cell proliferation and a strong immune response in mice, which is important for antiviral effects. The same group also demonstrated the highest levels of IFN-γ and IL-4 secretion. The results revealed a significant difference between NS3 + IL-12 and NS3 groups in the IFN-γ response, but not for IL-4. The finding indicates that IL-12 can increase cell immunity against HCV antigen, but it does not increase antibody production. Therefore, the HCV NS3 gene might be considered as a possible vaccine target (Pang P S, 2002).
Arribillaga et al. showed that the recombinant replicationdeficient adenoviral vector is able to efficiently express NS3 protein (RAdNS3) and induce specific humoral and cellular immunity. Mice immunized with the vector generate IFN-γ and IL-2-producing CD4+ cells in response to recombinant NS3 protein (Arribillaga L, 2002). In another study, Encke et al. showed that HCV NS genes are effective vaccine targets that induce cellular immunity and can protect tumor formation in immunized mice (Encke J, 1998). Accordingly, NS genes, including NS3, can induce antitumor cellular immunity.
Gao et al. showed that a pHCV-NS3-Th1 vaccine based on Class Ⅱ-associated invariant chain peptide (CLIP) genetic substitution resulted in high cellular proliferation and IFN-γ production. In pHCV-NS3-Th1-treated groups, CD4+ proliferation was significantly higher, but the pHCV-NS3 group failed to produce suitable IL-4 cytokine (Gao M, 2006). In accordance with this result, we found that mice immunized with the NS3 gene showed higher and lower IFN-γ and IL-4 secretion, respectively.
IL-12 is important for stimulating and establishing a population of CD8+ T cells with long-term memory (Kieper W C, 2001; Schmidt C S, 1999) and effectively activating CD8+ T cell responses to antigens (Scott P, 1997). Along with enhancement of cell-mediated immunity, IL-12 suppresses antibody production and attenuates the humoral immune response (Sin J I, 1999; Shan M, 2002). Therefore, IL-12 has potential as an adjuvant in prophylactic and therapeutic vaccines (Scott P, 1997).
Sin et al. reported that co-injection of glycoprotein D HSV-2 DNA vaccine with IL-12 leads to increased immune responses and better protection against HSV-2 in a mouse model. However, the production of IL-4, IL-10, and monocyte chemotactic protein-1 (MCP-1) was inhibited by IL-12 co-injection. In contrast, lymphocyte proliferative responses including secretion of cytokines (IL-2 and IFN-γ) and chemokines were significantly increased by IL-12 co-injection (Sin J I, 1999). Another study by Gherardi et al. demonstrated that co-injection of IL-12 with HIV type 1 (HIV-1) DNA vaccine can enhance cell-mediated immune responses against HIV (Gherardi M, 2000).
Shan et al. showed that co-delivery of IL-12 expression cassettes with vectors expressing HCV core, (pc), E1, and E2 in mice resulted in enhanced CTL responses and reduced specific antibody responses. The results demonstrated that administration of IL-12 as an adjuvant in DNA vaccine can induce cell immune responses by activating the Th1 pathway (Shan M, 2002). In conclusion, our findings show that HCV NS3 DNA vaccination in parallel with a genetic adjuvant shows promise for further vaccine development.













 DownLoad:
DownLoad: