-
Japanese encephalitis virus, a member of the Flavivirus genus in the family Flaviviridae, is the leading cause of epidemic Japanese encephalitis in the South-Eastern Asia, including China, Japan, Indonesia, Vietnam, Malaysia and India, with 62, 000 clinical cases reported annually (http://www.who.int/water_sanitation_health/diseases/encephalitis/en/) (Campbell G L, et al., 2011). JEV cycles among pigs and birds through transmission by Culex species mosquitoes. The mosquito is also the most important vector for human infection (Konno J, et al., 1966). JEV infection in human can cause fever, headache and other incapacitating manifestations involving neurological complications (Ghosh D, et al., 2009). Currently no specific treatment exists for patients infected with the virus. Only four types of JEV vaccines are used in different endemic regions (Hennessy S, et al., 1996; Hoke C H, et al., 1988), the inactivated mouse brainderived Nakayama or Beijing-1 strain, the inactivated cell culture-derived Beijing-3 or SA14-14-2 strain, the liveattenuated cell culture-derived SA14-14-2 strain, and the live YFV 17D chimeric vaccine (IMOJEV) (Appaiahgari M B, et al., 2010; Song B H, et al., 2012). Recently, an approved inactivated JEV vaccine, IXIARO (IC51), was found to be safe and immunogenic in a primary immunization schedule (Eder S, et al., 2011). However, due to licensing issues, safety and cost, their universal applications are limited (Nothdurft H D, et al., 1996; Plesner A M, P. Arlien-Soborg, and M. Herning, 1998; Schioler K L, et al., 2007).
Flaviviruses include many important infectious pathogens, including Japanese encephalitis virus (JEV), dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), tick-borne encephalitis virus (TBEV) and St. Louis encephalitis viruses (SLEV). The genomes of flaviviruses are plus-sense, single-stranded RNAs of 11kb in length, containing 5'-, 3'-untranslated regions (UTR) and a single open reading frame (ORF). The ORF encodes a long polyprotein which is co-or post-processed in the combination of viral and cellular proteases into three structural (Capsid [C], pre-membrane [prM] and envelope [E]) and seven nonstructural proteins (NS1, NS2A/B, NS3, NS4A/B and NS5) (Lindenbach B D, H. J. Thiel, and C. M. Rice, 2007). Nonstructural proteins act in viral replication and assembly, as well as in evasion of host innate immune responses (Lindenbach B D, H. J. Thiel, and C. M. Rice, 2007). Structural proteins, components of viral particles, play a critical role in viral assembly/release, receptor binding and entry. The capsid protein interacts with the viral genome to form the nucleocapsid (Jones C T, et al., 2003). prM acts in regulation of the structural changes of E protein by the heterodimeric association with E (Heinz F X, et al., 1994), which is very important for virus maturation (Yu I M, et al., 2008).
E protein, the major glycoprotein on the surface of virion (Kuhn R J, et al., 2002), plays a critical role in receptor binding (Chien Y J, et al., 2008; Lozach P Y, et al., 2005; Miller J L, et al., 2008), membrane fusion (Zaitseva E, et al., 2010), and neutralizing antibodies targeting (Nybakken G E, et al., 2005). Solution of the crystal structure of the JEV E protein ectodomain has revealed that JEV E protein is a head-to-tail homodimer with three structurally distinct domains: a central β-barrel (domain Ⅰ, DI), an elongated dimerization region (domain Ⅱ, DII), and a C-terminal immunoglobulin (Ig)-like module (domain Ⅲ, DIII) (Luca V C, et al., 2012). DIII is thought to contain the receptor-binding sites (Crill W D, et al., 2001) and major antigenic region recognized by neutralizing antibodies (Wu K P, et al., 2003). Structurally, DIII in WNV, DENV and JEV shows strong similarities with only marginal differences (Kanai R, et al., 2006; Luca V C, et al., 2012; Modis Y, et al., 2003; Rey F A, et al., 1995), particularly in areas that constitute virus neutralizing epitopes. DIII has been proposed as to be an efficient antigen for serologic diagnosis and therapeutics as well as crossprotection (Chavez J H, et al., 2010). Due to the close antigenic and genetic relationship between flaviviruses, humoral immune responses result in the production of the virus species-specific as well as cross-reactive antibodies (Calisher C H, et al., 1989; Tesh R , et al., 2002). It has been previously described that a unique mAb based on DENV2 DIII can neutralize and cross-react with all four DENV serotypes (Rajamanonmani R, et al., 2009). Interestingly, the WNV DIII protein has been used for inhibition of WNV and DENV2 viruses infection in C6/36 cells (Chu J J H, et al., 2005), demonstrating the structural similarities of some epitopes in DIII between WNV and DENV2.
In this study, we investigated the DIII proteins' ability to provide cross-protection against JEV infection. Initially, we purified the soluble envelope DIII proteins of JEV, DENV1-4 and WNV by using a denature-refolding procedure. Rabbits immunized with JEV DIII protein produced polyclonal antibodies which could recognize the recombinant JEV DIII efficiently, block the JEV infection and partially react with DENV3, DENV4 and WNV DIII. To some extent, all of the six flavivirus DIII proteins could inhibit the JEV infection in BHK-21 cells competitively. In addition, the mice immunized with the given DIII proteins could be protected from a lethal JEV challenge to varying degrees. The study suggests that recombinant flavivirus DIII proteins could be an alternative strategy for the development of vaccine and therapeutics against JEV infection.
HTML
-
Vero (African green monkey kidney) and BHK-21 (Baby hamster kidney) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The JEV strain P3 (GeneBank accession number U47032) was propagated in Vero cells in DMEM supplemented with 2% FBS and 1% penicillin/streptomycin. All the culture and virus propagation were incubated at 37 ℃ with 5% CO2. The virus was stored at -80 ℃.
-
The amino acid sequences corresponding to the E protein DIII region of the following viruses were downloaded from National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/): 571-680aa of DENV1 (strain FGANA d1d, GeneBank accession number AF226686), 571-680aa of DENV2 (strain New Guinea C, GeneBank accession number AF038403), 569-678aa of DENV3 (strain H87, GeneBank accession number M93130), 570-679aa of DENV4 (GeneBank accession number M14931), 584aa-693aa of WNV (strain NY99-flamingo382-99, GeneBank accession number AF196835), 587-696aa of JEV (strain SA14-14-2, GeneBank accession number AF315119). Multiple sequence alignment was carried out using GENEDOC program in the software CLUSTALW.
-
The plasmid constructions were performed according to the standard molecular biology procedures. The plasmids containing partial sequences of the viral genome were applied as templates for amplifying the fragment encoding DIII by PCR using specific primer pairs (Dou J L, et al., 2011). The corresponding primer pairs and templates were listed in Table 1. Afterwards, the purified PCR products were digested and inserted into the bacterial expression vector pET28a (Novagen, Darmstadt, Germany) using corresponding restriction enzyme EcoR I、Sal I or Xho I. All the constructs were confirmed by restriction enzyme digestion and DNA sequencing.

Table 1. Primers and templates for PCR used in this study
-
The positive colony was picked up and transferred into 5 mL LB medium with 50 μg/mL of kanamycin. The logarithmic phase culture was induced by adding IPTG (0.5 mmol/L) and incubating at 30 ℃ for 4-6 h. Afterwards, cells were harvested and resuspended in a lysing buffer (20 mmol/L Tris-HCl, pH7.4, 500 mmol/L NaCl), and lysed by sonication for 30 min on ice. The supernatant and pellet were collected separately and analyzed on 12% SDS-PAGE.
In order to harvest inclusion bodies, recombinant clones were incubated in 1L LB medium with 50 μg/mL of kanamycin. After induction and sonication, cells were pelleted down by centrifugation, and dissolved in a solubilization buffer (lysing buffer supplemented with 8 mol/L urea) on ice for 1 h, followed by centrifugation at 16, 000 × g for 30 min at 4 ℃.
The clear supernatants were then purified by using a nickel-affinity agarose column (GE, CA, USA). The columns were equilibrated with binding buffer (20 mmol/L TrisHCl, pH7.4, 500 mmol/L NaCl, 8 mol/L urea and 5 mmol/L imidazole. The supernatants were passed through the Ni-NTA columns, enabled to bind for 30 min, then washed with wash buffer (binding buffer containing 20 mmol/L imidazole). Later, proteins were eluted in an elution buffer (20 mmol/L Tris-HCl, pH 7.4, 500 mmol/L NaCl, 8 mol/L urea and 500 mmol/L imidazole), loaded in a dialysis bag and dialyzed overnight at 4℃ in 1 L of a dialyzing buffer (500 mmol/L Tris-HCl, 150 mmol/L NaCl, 10% Glycerol, pH 7.9). The purified soluble DIII proteins were analyzed by SDS-PAGE and stored at -80 ℃. Protein concentration was measured by using a BCA protein assay kit (Thermo, MA, USA) according to the manufacturer's instructions.
-
The inclusion bodies of JEV DIII proteins were denatured and purified by SDS-PAGE. The band corresponding to the calculated size of JEV DIII protein was cut, ground and mixed with phosphate buffered saline, to be used as an immunogen for polyclonal antibodies generation. Subsequent booster immunizations with about 0.5 mg mixture were administered to rabbit 14, 28 and 42 days following the primary injection. Ten days after the final injection, serum was prepared before antigenic stimulation and 10 days after the last stimulation, and used to detect DIII protein in Western blotting and ELISA assay. The ratios of S/N (S, D450nm of sample; N, D450nm of negative) more than 2.1 were considered as significant (Chow L, et al., 1992).
-
The purified soluble JEV DIII protein was diluted in a buffer (50 mmol/L Na2CO3, 50 mmol/L NaHCO3, pH 9.6) to a final concentration of 10 μg/mL, and coated to a 96-well microtiter plate at 37 ℃ for 2 h. The plate was washed three times with PBS, followed by blocking in 0.5% bovine serum albumin at 37 ℃ for 1 h. After twice washing with PBS, the plate was incubated with relevant polyclonal antibodies (10-fold dilutions) at 37 ℃ for 1 h. The plate was washed three times with PBS, followed by incubating with HRP-conjugated goat anti-rabbit lgG (1:2000 diluted) at 37 ℃ for 1 h. After another three washes, 1×TMB (Tetramethyl Benzidine) substrate (Invitrogen, CA, USA) was added and incubated at 30 ℃for 45 min. The reaction was stopped by addition of 0.5 mol/L HCl. The absorbance at 450 nm was quantified by a microplate reader (Bio-Tec, VT, USA).
-
The purified soluble DIII protein samples were mixed with 5×SDS sample buffer containing 0.05% 2-mercaptoethanol, heated at 95 ℃ for 5 min, and resolved on 12% SDS-PAGE gels. For Western blot, proteins were transferred to nitrocellulose membrane followed by one hour of blocking at room temperature in PBS (135 mmol/L NaCl, 2.7 mmol/L KCl, 1.5 mmol/L KH2PO4 and 4 mmol/L Na2HPO4, pH7.4) supplemented with 5% skim milk. Afterwards, the blots were incubated with rabbit polyclonal antibodies (1:2000 diluted) in PBS buffer at 37 ℃ for 1 h. After three washes (PBS containing 0.1% Tween-20), the blots were incubated with a goat anti-rabbit IgG conjugated with alkaline phosphatase (1: 5000 diluted, Millipore) at 37 ℃ for 2 h. After another three washes, the blots were developed with NBT/BCIP (Invitrogen).
-
PRNT assays were performed on a monolayer of BHK-21 cells grown with DMEM containing 10% FBS at 37℃ with 5% CO2. Serially diluted antibody (50 μL) was mixed with 100 PFU of JEV strain P3 in BA-1 with 2% FBS. BA-1 contains M199 with Hanks' salts and l-glutamine, 0.05 mol/L Tris, 1% bovine serum albumin, 0.35 g/L sodium bicarbonate, 100 units/mL penicillin, 100 units/mL streptomycin, and 1 µg/mL fungizone, pH 7.4. The mixtures were incubated at 37 ℃ for 1 h to form the virus-antibody complexes, and added to each well of 24-well plate. After 1 h adsorption at 37 ℃, unabsorbed viruses were thoroughly removed by washing three times with cold PBS. Subsequently, the cells were incubated with DMEM plus 2% FBS, 1% methylcellulose (Aquacide II, Calbiochem). After four days of incubation at 37 ℃ with 5% CO2, the cells were fixed with 3.7% formaldehyde and stained with 1% crystal violet. The number of plaques was counted and normalized to the number of plaques derived from control wells in which virus was mixed with pre-immunized sera.
-
This assay was performed as described previously with a few modifications (Chien Y J, et al., 2008). BHK-21 cells (2 × 106 cells per well) were seeded in a 6-well plate. 24 h postseeding, soluble recombinant DIII proteins or BSA (as a negative control) were diluted with assay buffer (DMEM, 2% FBS) to a concentration range of 25, 50 and 100 μg/mL, and incubated with the cells in culture medium at 4 ℃ for 1 h. Unabsorbed proteins were thoroughly removed by three washes with cold PBS. Afterwards, each cell was infected by 100 PFU JEV at 37 ℃ for 1 h with shaking at every 15 min. After 1 h incubation, the cells were completely washed three times with PBS to get rid of unbounded viruses, the inoculums were replaced with DMEM plus 2% FBS, 1% methylcellulose (Aquacide II, Calbiochem). After four days of incubation at 37 ℃ with 5% CO2, the wells were fixed with 3.7% formaldehyde and stained with 1% crystal violet.
-
Seven independent groups of five 3-week-old female BABL/c mice were used. Each group of mice was given intraperitoneal injections respectively with 25 μg purified recombinant JEV DIII, DENV 1-4 DIII, WNV DIII or PBS. The samples mixed with Freund's complete adjuvant (Sigma, Mo, USA) adjuvant were used to enhance the response to the immunogen for the first time, followed by boosted thrice in Freund's incomplete adjuvant (Sigma) at two-week intervals. The mixture was homogenized by polytron until stable water-in-oil emulsion was obtained. Two weeks after the final boosting, mice were injected intraperitoneally with 1 × 107 PFU of the JEV strain P3. Mice were observed daily for three weeks. All mice experiments were carried out according to the protocols approved by the Animal Experiment Committee of Chinese Academy of Science, Wuhan Institute of Virology.
Viruses and cells
Amino acid sequence alignment
Construction of recombinant plasmids
Expression and purification of recombinant DIII proteins
Generation of polyclonal antibodies against JEV DIII
Enzyme-linked immunosorbent assay (ELISA)
SDS-PAGE and Western blot
Plaque reduction neutralization test (PRNT)
Inhibition of JEV infection by soluble recombinant DIII proteins
Protection of mice from lethal JEV challenge
-
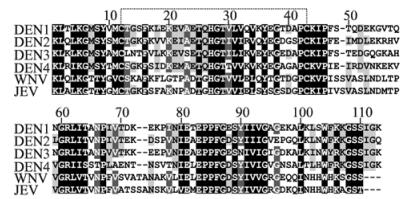
Multiple sequence alignment of DIII of JEV and other flaviviruses (DENV1-4, JEV and WNV) revealed several identical regions among them, as shown in Fig. 1. A conserved disulphide bond is shared among all the given DIII regions, the DIII of WNV is closest to that of JEV with 72% identical residuals and the homology between DENV2 and JEV DIII is 32%. The DIIIs of DENV1, 3 and 4 are very different from that of JEV with 18%-26% identical residuals.
-
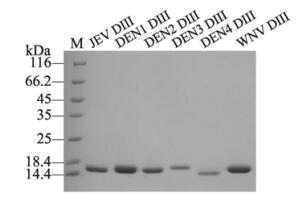
The JEV DIII was efficiently expressed in E. coli when induced with 0.5 mmol/L IPTG (Lane 2 in Fig. 2), but most of the JEV DIII protein formed inclusion bodies and precipitated in lysis buffer (Lane 3 in Fig. 2). The inclusion bodies were then dissolved in lysis buffer supplemented with 8 mol/L urea followed by purification with Ni column. After the final refolding step by gradually removing the urea, we successfully obtained the soluble forms of JEV DIII with a calculated size of ~13kDa (Lane 4 in Fig. 2). The soluble DIII proteins for DENV1-4 and WNV were purified with the same denature-refolding procedure (Fig. 3). All of the calculated sizes for DENV1-4 and WNV are about 12-14kD.

Figure 2. Expression and purification of JEV DIII. Portfolio of purification of JEV DIII proteins. Lane M, Molecular mass markers; Lane 1, total proteins from E.coli BL21 before induction by IPTG; Lane 2, total proteins after induction with IPTG; Lane 3, including bodies post sonication; Lane 4, purified proteins after dialyzing. The arrow indicates the bands corresponding to the calculated size of JEV DIII protein.
-
The serum against JEV DIII was obtained by immunizing rabbit with denatured JEV DIII proteins. Our ELISA data indicated that purified JEV DIII protein could be recognized efficiently by rabbit serum generated from denatured JEV DIII.As shown in Fig. 4A, the titer of neutralizing antibodies derived from JEV-DIII protein was as high as 105. Western blot indicated that the JEV DIII could be easily detected by the rabbit serum with a dilution of 1:1000 (Fig. 4B), which was consistent with our ELISA results. Interestingly, the serum could cross-react with the soluble DIII of DENV3, DENV4 and WNV but not DENV1 and DENV2, indicating the similarities and variations of the DIII among different flaviviruses (Fig. 4B). PRNT assay revealed that a dilution of 1:8 of the rabbit serum could completely abolish the JEV infection, the dilution of serum for PRNT50 would then reach 1:340 by calculation (Fig. 5).The data demonstrated that the JEV DIII had high immunogenicity to produce neutralizing polyclonal antibodies against JEV infections in cells.

Figure 4. Characterization of reactivity of rabbit polyclonal antibody against flaviviruses DIII. A: Titration analysis of rabbit polyclonal antibodies using ELISA. S/N≥2.1 (S, D450nm of sample; N, D450nm of negative) considered as significant. B: Cross-reactivity of rabbit polyclonal antibody against flaviviruses DIII. Western blot was performed using rabbit polyclonal antibodies against JEV DIII (1:2000 diluted) (see details in Materials and Methods). Around 3 µg purified DIII proteins were loaded in each lane and resolved in 12% SDS-PAGE. The species of DIII are labeled on the top of the panel.
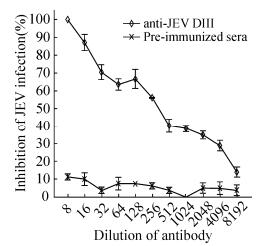
Figure 5. Plaque reduction neutralization testing (PRNT) analysis of rabbit antibodies against JEV infection. The rabbit antibodies were generated by immunization of rabbit with denatured JEV DIII (see details in Materials and Methods). The BHK-21 cells were incubated with indicated mixture with JEV (100 pfu/well) and dilutions of rabbit serum against JEV or pre-immunized serum. Viral titers were measured by plaque assay. The mean value from three independent experiments is presented and the error bar indicates the standard deviations (n=3).
-
The similarities of the amino acid sequences between those flaviviruses, as well as the cross-reactivity of rabbit serum generated by JEV DIII against other flavivirus proteins, suggested certain common epitopes among the DIII of flaviviruses. To validate this hypothesis, we therefore performed a competitive inhibition assay of JEV infection in BHK21 cells using given soluble recombinant DIII proteins. As expected, JEV DIII suppressed JEV infection significantly in a dose-dependent pattern. Furthermore, the ability of other flaviviruses DIII to inhibit JEV infection varied in the following order (from stronger to weaker): WNV≥DENV4 > JEV≥DENV2 > DENV1 (Fig. 6), occurs in a dose-dependent manner. Remarkably, competitive inhibition of JEV entry into BHK cells with the purified soluble WNV and DENV4 DIII proteins was slightly higher than for JEV DIII. In contrast, bovine serum albumin (BSA) showed no negative effect on JEV infection at various concentrations.
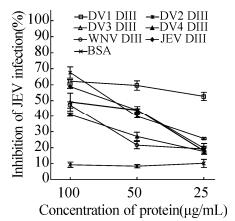
Figure 6. Cross-protection of JEV infection in vitro using recombinant DIII proteins. Competitive inhibition of JEV infection with recombinant DIII proteins. BHK-21 cells were pretreated with indicated concentrations of recombinant DIII proteins and subsequently infected with JEV (100 PFU). Viral titers were determined by plaque assay after 4 days incubation. Averages and standard deviations from three independent experiments are presented.
-
To observe the cross-protection of recombinant DIII against JEV infection in vivo, we immunized each group of three-week-old female BABL/c mice with equal mass of DIII proteins, and monitored the morbidities and mortalities of mice challenged with JEV P3. In Fig. 7, as expected, all the mice injected with JEV DIII protein got complete protection from the JEV infection. As listed in Table 2, the level of protection in test groups immunized with soluble DENV2 or WNV DIII was also significant (p=0.0018) when compared with other groups during the tracing periods, showing 100% protection against JEV challenge. The survival rate of mice immunized with DENV3 and DENV1 DIII group was 80% and 60% respectively but DENV4 DIII protein only conferred 40% protection. Interestingly, the group of DENV4 DIII protein immunized mice showed more severe encephalitis symptoms than that of JEV, DENV1 and DENV3 group. As a control, the mice immunized with PBS had no protection from JEV infection. Our data here shows the result that soluble DIII from heterogeneous flaviviruses could be used to inhibit JEV infection in vivo.
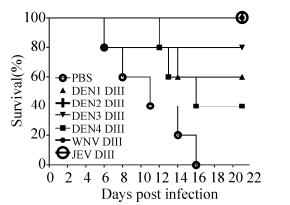
Figure 7. Cross-protection of JEV-infected mice using recombinant DIII proteins. 3-week-old female BALB/c mice (five mice per group) were immunized by an intraperitoneal route using soluble flavivirus DIII proteins or PBS (as negative controls) four times at two-week intervals, and subsequently challenged with lethal JEV viruses (107 PFU). The mortalities of each group were monitored every day over a period of 21 days post infection. The calculated survival percentages are presented.

Table 2. Challenge studies in immunized mice
Amino acid sequence alignment
Expression and purification of recombinant JEV and flavivirus envelope DIII protein
Validation of polyclonal antibodies against JEV-DIII
Competitive inhibition of JEV infection with soluble recombinant DIII proteins
Protective immunity against lethal JEV infection in BALB/c mice
-
The DIII domain in the flavivirus E protein is an immunoglobulin-like domain which is thought to be involved in the virus receptor-binding process and is recognized by virus-neutralizing antibodies. No vaccine is available for most flaviviruses, but DIII can induce neutralizing antibodies and immune responses, suggesting its possibility as a potential candidate in vaccine development. In the present study, DIII of JEV E protein fused with a six histidine tag was synthesized in E. coli. Refolded JEV-DIII in combination with Freunds complete/ incomplete adjuvant was administrated in mice as a vaccine which could induce immunomodulatory effects with high protection. As a consequence, the polyclonal antibodies induced by JEV-DIII linear antigens demonstrate strong capability to neutralize JEV efficiently and consistently in vitro (Fig. 5), suggesting partial virus-neutralizing epitopes may be distributed on the external surface of JEV-DIII. Antibodies from denatured JEV-DIII could induce strong neutralizing antibodies with high efficiency, preventing JEV from binding to the respective cellular receptor. These findings indicate the possibility of generating JEV-DIII specific antibodies in a straightforward manner and on a large scale, necessary requirements for a commercial vaccine.
In order to examine the immunogenicity and crossprotection efficacy of the soluble recombinant flavivirus DIII proteins, we challenged mice with the JEV P3 strain in each immunized group. Our data showed that most mice were protected against lethal infection, which implies that the majority of the cross-reactive antibodies recognized epitopes involving the conversed residues in domain Ⅲ. Previously Chu J H et al. reported the immunization of proper folding WNV-DIII was effective at preventing WNV infection, protecting > 90% of challenged mice. It is certain that cross-reactions exist between WNV and JEV as immunization with JEV-DIII induces cross-protective immunity against WNV infection (Li S H, et al., 2011). Due to the high similarity of the primary structure between WNV, JEV-DIII and DENV2 DIII proteins, they all displayed 100% protection against JEV infection in animal assay. Although cross-reactive immune responses have been reported, our results indicate a significant similarity in immune responses, demonstrating that soluble recombinant flavivirus DIII proteins possess a broad dose-dependent inhibition in vitro and cross-protective immunity in lethal JEV infection in vivo. The antibody may protect mice through multiple mechanisms such as viral neutralization and complement activation. To investigate the protective immunity of DIII, further studies about the neutralizing antibodies titers of immunized mice would be needed. To explore whether the DIII could induce cell-mediated immune and humoral immune responses, more evidence about the antibody levels are needed, including investigation of various cytokines expressed in immunized mice to provide the basis for the protection results.
On the other hand, with a view to use as a potential candidate in therapeutics, previous reports have indicated that flavivirus DIII can compete with the virus during entry by preventing viral binding to related receptors on the cell surface (Chu J J H, et al., 2005). The protection and cross-reactivity of DIII to the flaviviruses infection are dependent on the similarities on both primary and secondary structure. Despite these similarities, each flavivirus displays a unique surface to bind antibodies. Several studies showed that DENV and WNV DIII binding on the surface of host cells even between different serotypes (Chu J J H, et al., 2005). We demonstrated that exogenously adding properly folding JEV DIII could inhibit the JEV infection. The antagonistic effects of other soluble flavivirus DIII on JEV infection are shown in Fig. 4A, and functional analysis revealed that purified flavivirus DIII blocked the entry of JEV into BHK cells, indicating that the recombinant proteins fold properly. More information is needed to determine whether similar attachment or receptor molecules are utilized by flaviviruses in host cells. We suggest that the domain involved in virus attachment and assisting binding to surface receptors on BHK cells may be common among the viruses, and provide a route to a specific drug-target common to the flavivirus family.
To conclude, recombinant domain Ⅲ of the flavivirus DIII proteins were expressed, purified and proved to be biologically functional in vitro and in vivo. These peptides could serve as a potential target for the design of anti-viral agent development, having a broad application for the prevention and treatment of infection with flavivirus. The method described in this study may help development of improved vaccines. While the potential benefit of flavivirus DIII vaccination should be considered in view of the great risk of emerging JEV, future work is still required before final goal of highly effective vaccines can be achieved.













 DownLoad:
DownLoad: