-
The rapid propagation of multidrug resistant bacterial strains is leading to renewed interest in bacteriophage therapy. With challenges in the treatment of bacterial infections, it is essential for people worldwide to understand how alternative approaches, such as bacteriophages, could be used to combat antibiotic resistant bacteria. The Eliava Institute of Bacteriophages, Microbiology and Virology (Tbilisi, Georgia) is arguably the most famous institution in the world focused on the isolation, study, and selection of phages active against a variety of bacterial pathogens.
HTML
-
The Institute of Bacteriophages in Tbilisi was founded by Georgian microbiologist Giorgi Eliava and French Canadian microbiologist and phage discoverer, Felix d'Herelle in 1923 (Figure 1A). This facility later named the George Eliava Institute of Bacteriophages, Microbiology and Virology (Figure 1B), which remains the current name. Although in past decades, the Eliava Institute has undergone many changes, phage research and phage therapy have remained the principal activities of the institute.

Figure 1. (A) Felix d'Herelle and George Eliava working at the bacteriophage institute in Tbilisi in the 1930s. (B) The George Eliava Institute of Bacteriophages, Microbiology, and Virology. (Courtesy of the Eliava Institute)
The research conducted at this institute is focused on the use of bacteriophages in the following areas: human therapy and prophylaxis, veterinary applications, food safety, and environmental protection. Particular current attention is devoted to the isolation and selection of effective bacteriophages against antibiotic-resistant bacterial strains.
-
A large collection of phages and pathogenic bacterial strains has been relentlessly compiled at the institute. The Eliava Analytical–Diagnostic Center provides the detection and identification of bacterial and viral pathogens, which are the causative agents of infectious diseases. Together with antibiotic-susceptibility tests, the center also performs phage-susceptibility tests. Another part of the Institute, Eliava Biopreparations, produces several phage preparations for treatment and prophylaxis: Pyophage (phages against Staphylococcus aureus, Escherichia coli, Streptococcus spp., Pseudomonas aeruginosa, Proteus spp.); Intesti phage (Shigella, Salmonella, entero-pathogenic E. coli, Proteus, Enterococci, Staphylococci, and P. aeruginosa); Enkophage (Salmonella spp., Shigella spp., entero-pathogenic E. coli, Staphylococcus spp.); SES phage (Staphylococcus spp., Streptococcus spp., E. coli); Fersis (Staphylococcus spp., Streptococcus spp.); and Staphylococcal monophage (Figure 2). The preparations developed in Tbilisi have been studied through extensive characterization as well as through preclinical and clinical trials.
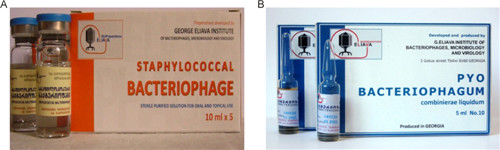
Figure 2. hage preparations produced by the Eliava Biopreparations for treatment and prophylaxis. (A) Staphylococcal bacteriophage. (B) Pyo-Phage (phages against Staphylococcus aureus, Escherichia coli, Streptococcus spp., Pseudomonas aeruginosa, Proteus spp.). (Courtesy of the Eliava Institute)
Many people from various parts of the world currently express their willingness to undergo phage treatment against different infections, including those that are caused by antibiotic-resistant bacterial pathogens. From 2012 to December 2014, the Eliava Phage Therapy International Center has received 5, 205 patient visits. Among these, 3, 290 visits were for phage treatment. Phage preparations were also sent to another 130 patients abroad. Thirty-nine foreign patients were treated at the Center (from France, Canada, USA, Romania, and Norway). Frequently observed diseases in our clinics include those in urology, gynecology, internal medicine, and pediatrics. The overall outcome of these treatments is exciting with more than 95% exhibiting signifi cant improvement and recovery. In no cases were there complications or side effects after phage application.
Phage preparations are used in acute as well as in chronic patients. In chronic cases, the main advantages of phage treatment are in reducing the necessity for antibiotic use in complex therapies by 50% and in prolonging remission period (infection-free periods between colonization episodes).
-
The Eliava Institute has developed new, phage-based products and technological approaches for their effective, quality controlled production. Strong collaboration with the medical community in the design of clinical trials according to international standards is absolutely critical in supporting the broader implementation of phage therapy. The next step at the Eliava Institute is to commercialize phage-based products following international standards; to extend initiatives for the elaboration of new phage preparations; and to explore the most appropriate organizational development for broader production, application, and marketing of its biological preparations.













 DownLoad:
DownLoad: