-
Rabies virus (RABV) is a single-stranded negative sense RNA virus of the genus lyssavirus with a distinctive bullet-shape structure in the rhabdoviridae family. RABV is a neurotropic virus, which causes acute inflammation of the brain in humans and other mammals with a case fatality rate of almost 100% (Fooks et al., 2014). Fortunately, rabies is a preventable viral disease by prompt vaccination. However, there are still approximately 59,000 human deaths reported each year all over the world, and most of these deaths occurred in Africa and Asia and associated with dog bites (Hampson et al., 2015). A latest epidemiological study demonstrated that most human rabies cases in China were reported in rural areas in the southern and eastern provinces where the dog immunization coverage of rabies was very low (Tan et al., 2017). Therefore, it is necessary to eliminating the rabies by vaccinating dogs to prevent rabies transmission from dog to human. It has been proposed that vaccination coverage of 70% of the canine population could efficiently reduce rabies transmission from dog to human (Hu et al., 2009). Live-attenuated rabies vaccines have been explored as a promising alternative to control rabies (Zhu and Guo, 2016). Recombinant RABV expressing a cytokine or chemokine have been demonstrated in our previous studies that could enhance the induction of virus-neutralizing antibody (VNA) and provide a higher survivorship after lethal viral challenge (Zhao et al., 2010; Wen et al., 2011; Zhou et al., 2013; Zhang et al., 2016b; Wang et al., 2017). Hence, over expressing a cytokine with immunoregulatory function is an efficacious strategy to develop a more efficacious rabies vaccine.
IL-15 is a member of gamma chain receptor cytokine family along with IL-2, IL-4, IL-7, IL-9, and IL-21. It is widely expressed by many cell types such as monocytes, macrophages, and dendritic cells (DCs) (Patidar et al., 2016). In addition, IL-15 is also a master regulator that links the innate and adaptive immune system. It plays a crucial role in the development, homeostasis, and function of T, NK, and NK-T cells, and is required for various functions of the B cells, DCs, macrophages, and mast cells as well (Waldmann and Tagaya, 1999). Previous studies showed that IL-15 could be used for cancer therapy (Pagliari et al., 2013), increasing antitumor activity (Tosic et al., 2014) and the production of IFN-γ to enhance the protective immunity against influenza virus, herpes simplex virus, Toxoplasma gondii and many other pathogens (Fawaz et al., 1999; Kutzler et al., 2005; Eickhoff et al., 2011; Perera et al., 2012). Furthermore, it was found that IL-15 could promote DCs maturation and induce a prevalent seroconversion to Th2-dependent antibodies when used along with staphylococcal enterotoxin B, suggesting that IL-15 has the potential for using as an adjuvant to enhance antibody responses (Saikh et al., 2008).
Recently, it was demonstrated that combined IL-15 and IL-21 as the adjuvant for DNA vaccine against Toxoplasma gondii could induce humoral response and cellular immune responses (Chen et al., 2014). Our previous study demonstrated that recombinant RABV expressing IL-21 could enhance immunogenicity through activating T FH and GC B cells rather than promoting the maturation of DCs (Zhang et al., 2016b). Therefore, to further characterize the role of IL-15 in RABV immunogenicity, the rRABV expressing IL-15 was constructed and investigated. The results indicated that expression of IL-15 could enhance the immunogenicity of RABV by promoting the maturation of DCs and the generation of T FH cells, GC B cells, and plasma cells in immunized mice.
-
BSR cells, a cloned cell line derived from BHK-21 cells, were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Mediatech) containing 10% FBS (Gibco, Grand Island, NY). LBNSE, a recombinant RABV (rRABV), is constructed from SAD-B19 strain (Conzelmann et al., 1990; Rasalingam et al., 2005) with mutations of the G at amino acid (aa) positions 194 and 333(Wen et al., 2011). The challenge virus standard 11 (CVS-11) and 24 (CVS-24), two pathogenic RABV strains, were propagated in NA cells and suckling mouse brains, respectively. Fluorescein isothiocyanate (FITC)-conjugated antibodies against the RABV N protein was purchased from FujiRab (Malvern, PA). Antibodies used for flow cytometric analysis, such as anti-CD11c (clone N418), CD80 (clone 16-10A1), CD86 (clone GL-1), I-A/I-E (clone M5/114.15.2), CD4 (clone GK1.5), CD185 (clone L138D7), CD279 (clone RMP1-30), B220 (clone RA3-6B2), GL7 (clone GL7), CD138 (clone 281-2) were purchased from eBioscience (San Diego, CA), and CD95 (clone 15A7) from Biolegend (CA San Diego). Six weeks old female BALB/c and ICR mice were purchased from the Center for Disease Control and Prevention of Hubei Province.
-
The LBNSE-IL15 cDNA clone was generated based on pLBNSE vector as described previously (Wen et al., 2011). Briefly, pLBNSE was digested with BsiW I and Nhe I between the G and L genes, and IL-15 gene was cloned and inserted into pLBNSE by using these two restriction sites. Two primers were used for amplifying IL-15 gene (forward primer: 5′- TGCTCGTACGA TGGAGAGGACCCTTGTC-3′ and reverse primer: 5′-GCTAGCTAGCCTAGGAGAGATGCTGATG-3′, BsiW I and Nhe I sites were underlined).
-
LBNSE-IL15 was rescued as described previously (Wen et al., 2011). Briefly, 2.0 μg of the full-length infectious clone, 0.5 μg of N helper plasmid, 0.25 μg of P helper plasmid, 0.1 μg of L helper plasmid, 0.15 μg of G helper plasmid were transfected into BSR cells using the SuperFect transfection reagent (Qiagen, Valencia, CA) according to the manufacture’s protocol. After 4 days incubation at 34 °C, the culture medium was discarded and fresh medium replenished for further 3 days incubation. After the incubation, the cell culture medium was harvested, and the rescued virus examined with FITC-conjugated anti-RABV N antibodies.
-
BSR cells were infected with LBNSE, LBNSE-IL15, or mock infected with the same volume of DMEM at multiplicity of infection (MOI) = 1. Then, 20 μL of TMS (Cell Titer 96 AQueous One Solution Cell Proliferation Assay) was added to the infected BSR cells at 1, 2 and 4 days post infection and incubated at 37 °C for 2 h. Then the plate was read at 490 nm by using the Microplate Reader (Molecular Devices, China).
-
Virus titration was performed with the direct fluorescent antibody assay as describe previously in BSR cells (Zhou et al., 2013). BSR cells in a 96-well plate were inoculated with serial 10-fold dilutions of virus and incubated at 34 °C for 48 h. The cell culture medium was discarded, and the cells were fixed with 80% ice-cold acetone for 30 min and then were washed twice with PBS, finally the cells were stained with FITC conjugated anti-RABV N antibody at 37 °C for 45 min. Antigen-positive foci were counted under a fluorescence microscope (Zeiss, Germany) and virus titer was calculated as fluorescent focus units per milliliter (FFU/mL). All titrations were carried out in quadruplicate.
-
The supernatant was subjected to an enzyme-linked immunosorbent assay (ELISA) to quantify the amount of IL-15 in cell culture supernatants by using the Mouse IL-15/IL-15R Complex ELISA Ready-SET-Go!® (San Diego, CA) according to the manufacturer’s protocol. Evaluation of antibody responses by ELISA, Groups of 10 female ICR mice aged 6 weeks were immunized intramuscularly (i.m.) with a single dose of 106 FFU/mouse of LBNSE, LBNSE-IL-15, or DMEM. Blood was collected weekly from 1 to 5 weeks post immunization and sera were isolated for analysis. RV protein- specific IgG, IgG1, IgG2a, IgG2b, and IgM antibodies were determined by ELISA and the sera were diluted at 1:6400, 1:30, 1:800, 1:1600, 1:30 respectively. RABV virus protein as capture antibody, other protocol is as the same as ELISA Kit.
-
Groups of 10 female ICR mice aged 6 weeks were immunized with a single dose of 106 FFU/mouse of LBNSE, LBNSE-IL15 or mock immunized with the same volume of DMEM by i.m. route; peripheral blood samples were collected weekly from 1 week post immunization (wpi) to 7 wpi and sera were isolated for analysis. VNA titers were measured using the fluorescent antibody virus neutralization (FAVN) test. Briefly, 50 μL of serial 3-fold dilutions of test serum and standard serum were prepared on 96-well microplates in 100 μL volumes. Each sample was added to four adjacent wells. A 50 μL volume of volume of CVS-11 suspension, containing 50–200 FFU, was added to each well. Then the microplates were incubated at 37 °C for 1 h in an incubator with 5% CO 2. Following incubation, 50 μL of the cell suspension, containing 2 × 10 4 BSR cells, was added to each well, and the microplates were incubated at 34 °C in an incubator with 5% CO 2 for 60 h. After the incubation, the plates were fixed with 80% ice cold acetone at –20 °C for 30 min. Cells were then stained with FITC-conjugated anti-RABV N antibodies for 45 min at 37 °C. After washing three times with PBS, the results were assessed by using an Olympus IX51 fluorescence microscope. VNA titers were expressed in IU/mL by comparison with the titer of a reference serum (obtained from the National Institute for Biological Standards and Control, Herts, UK) included in each test.
-
Groups of 25 ICR mice (Female, 6 weeks old) were immunized with 106 FFU of LBNSE, LBNSE-IL-15, or mock immunized with the same volume of DMEM by i.m. route. At 21 days post immunization (dpi), mice were intracerebrally (i.c.) challenged with 50 mouse intracerebral lethal dose 50 (MICLD 50) of CVS-24 in volume of 30 μL. The mice were then observed daily for 3 weeks.
-
To determine whether the expression of IL-15 could affect the pathogenicity of RABV in vivo, groups of 6 weeks old female ICR mice (10 per group) were infected with 2 × 10 6 FFU of LBNSE-IL15 or LBNSE, or mock infected with the same volume of DMEM by i.c. route. The infected mice were monitored twice daily for 14 days. The body weights was measured, and the development of diseases and death was recorded.
-
Groups of BALB/C mice (Female, 6 weeks old) were immunized by i.m. route with 106 FFU of LBNSE, LBNSE-IL-15, or the same volume of DMEM, and the draining lymph nodes, peripheral blood, spleen and bone marrow were collected at indicated time points. For DCs detection, the draining lymph nodes and peripheral blood were collected at 3 and 6 dpi; for T FH, GC B cells detection, the lymph nodes and/or spleen were collected at 7 and 14 dpi; the bone marrow was harvested for plasma cells detection at 7 and 14 dpi. Flow cytometry was carried out as described previously (Zhang et al., 2016b). Briefly, the lymph nodes, spleen, and bone marrow were homogenized to single-cell suspensions via a 40 μm nylon filter. For the blood samples, the red blood cells were lysed with ACK lysis buffer (BioSource International, Inc., Camarillo, CA) for 1 min at room temperature. The prepared single-cell suspensions were then stained with fluorescence-conjugated antibodies diluted in 0.2% bovine serum albumin (BSA) for 30 min at 4 °C in the dark. After incubation, cells were washed twice in PBS containing 0.2% BSA and fixed in 1% paraformaldehyde in PBS for 30 minutes. Then the samples were performed on Cytoflex flow cytometer (Beckman) and data analyzed by Cytoflex (Beckman) and FlowJosoftware (Tree Star).
-
Kaplan-Meier survival curves were analyzed by the log rank test; the other data statistical analyses were determined by an unpaired, two-tailed t test; the following notations indicate significant differences between different groups for all experiments: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
-
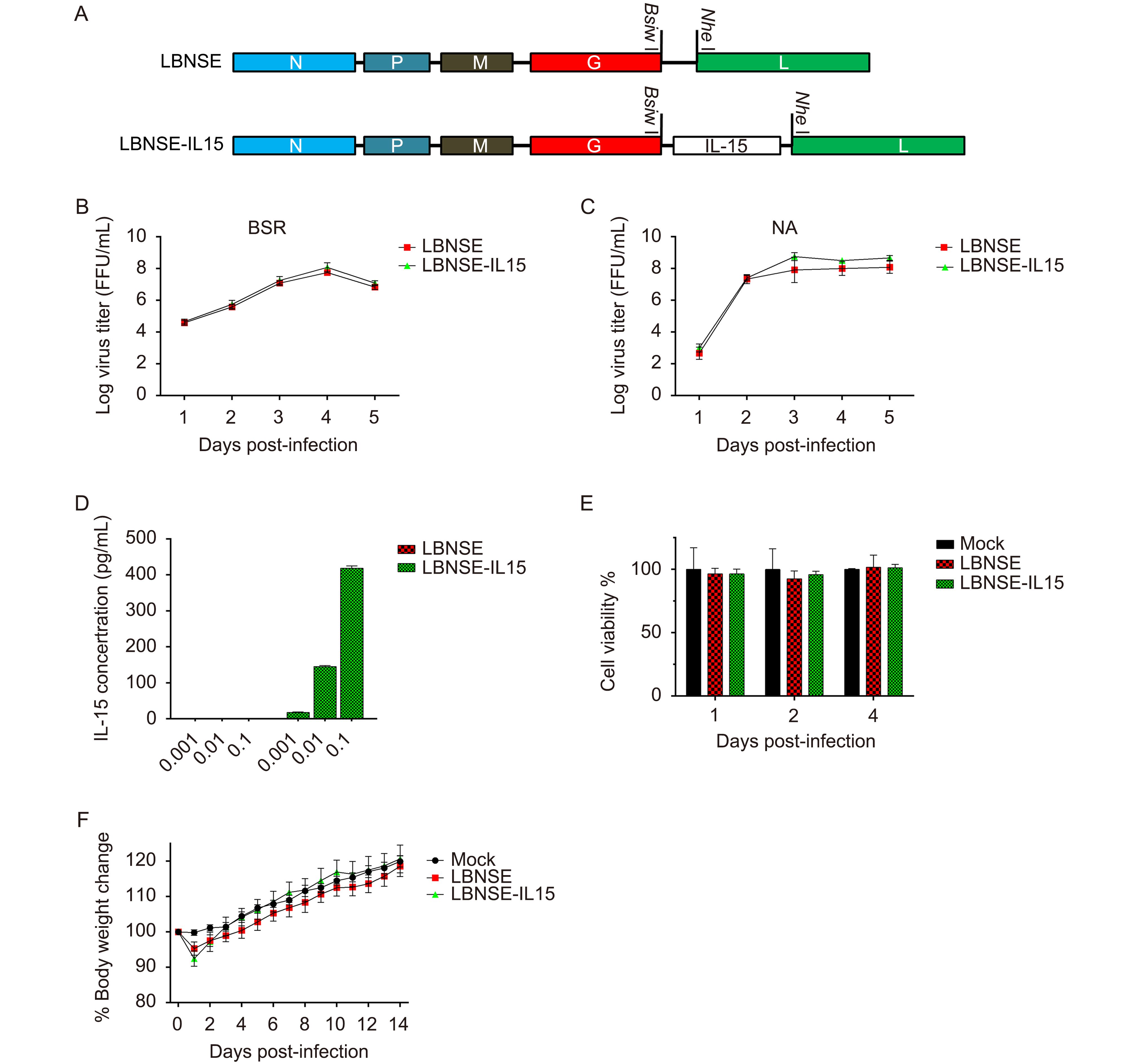
Our previous studies indicate that recombinant RABV expressing chemokine MIP-1 alpha (Zhao et al., 2010) or cytokine GM-CSF (Wen et al., 2011) could activate DC to enhance the innate and adaptive immune response after immunization. In order to investigate the role of another cytokine IL-15 as an adjuvant on the rabies-vaccine, IL-15 was cloned into LBNSE genome (Figure 1A) as described previously (Wen et al., 2011). LBNSE, based on SAD-B19 strain with two mutations in G protein at amino acid position 194 and 333, which can result in the virus nonpathogenic for adult mice after intracranial infection (Conzelmann et al., 1990; Wen et al., 2011). Insertion of the mouse IL-15 gene was confirmed by sequencing; the LBNSE expressing IL-15 was rescued and designated as LBNSE-IL15. In order to characterize the RABVs in vitro, viral growth kinetics were depicted in BSR and NA cells at a MOI of 0.01 as shown in Figures 1B and 1C, respectively; there was no significant difference was observed between LBNSE-IL15 and the parent virus LBNSE, indicating that the viral replication did not affected by the expression of IL-15. Moreover, the production of IL-15 in LBNSE-IL15 infected cells was detected by ELISA assay. As shown in Figure 1D, BSR cells infected with LBNSE-IL15 could produce IL-15 in a dose dependent manner, while the amount of IL-15 in LBNSE infected BSR cells was under detectable level. The cell viability after virus infection was also determined as described in Method section, and it was found that the cell viability of BSR cells was not affected by LBNSE-IL15 or LBNSE infection as shown in Figure 1E.
Furthermore, to determine if expression of IL-15 by RABV could affect the pathogenicity in vivo, groups of 10 ICR mice (6 to 8 weeks old, female) were injected with 2 × 10 6 FFU of LBNSE or LBNSE-IL15 or the same volume of DMEM by i.c. route. The body weights and clinical symptoms of infected mice were recorded daily for 2 weeks. No clinical signs were observed in all mice, and the body weight losses of LBNSE-IL15 infected mice were similar with those infected with LBNSE as shown in Figure 1F, suggesting that expression of IL-15 by RABV did not affect the viral pathogenicity in mice.
-
To determine whether the expression of IL-15 by LBNSE could affect the VNA induction after immunization, groups of mice were immunized with 106 FFU of LBNSE or LBNSE-IL15, or mock immunized with the same volume of DMEM by i.m. route. Blood samples were collected every week until 7 weeks post immunization (wpi), and sera were separated for the VNA titer detection by FAVN. As shown in Figure 2A, significantly higher levels of VNA were detected in mice immunized with LBNSE-IL15 than those immunized with parent virus LBNSE from 3 to 5 wpi. The dynamics of geometric mean titers (GMT) of VNA was also presented, and it was found that the highest GMT of VNA in LBNSE-IL-15 and LBNSE immunized mice were 32.17 IU/mL and 18.85 IU/mL induced at 5 wpi and 4 wpi, respectively (Figure 2B).
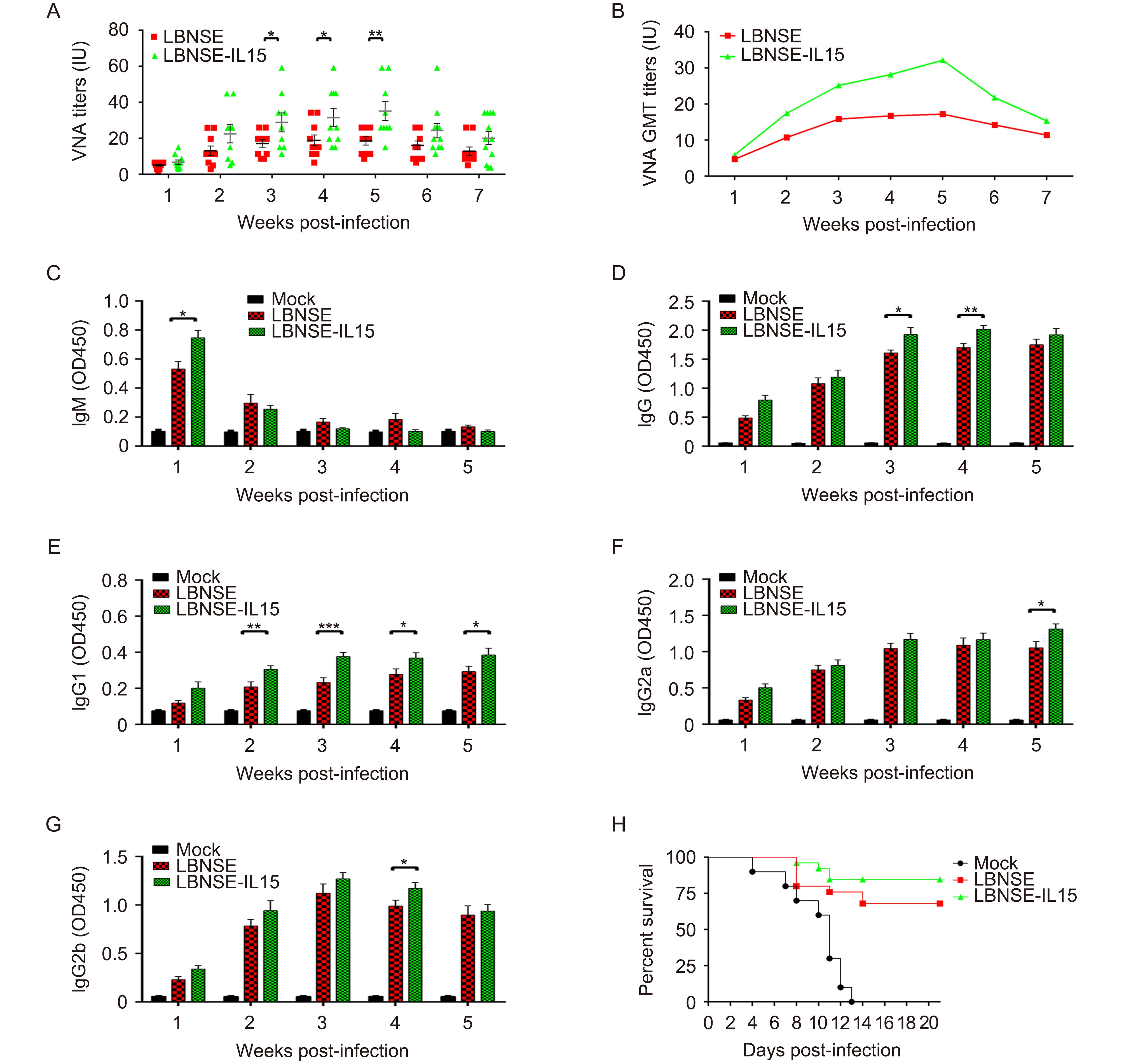
Figure 2. Expression of IL-15 by rRABV increases the production of virus-neutralizing antibody and improves the survivorship against pathogenic RABV challenge in immunized mice.
Moreover, the antibody isotypes (IgG and IgM) and IgG subclasses (IgG2a, IgG2b, and IgG1) were determined in sera collected from immunized mice by ELISA, and the results were as shown from Figures 2C–2G. As is known that IgM-to-IgG class switching occurs in the perifollicular regions during the early stages of B cell activation and development, therefore, significantly higher level of IgM was only detected at 1wpi (Figure 2C). Significant higher levels of RABV IgG were detected in LBNSE-IL15 immunized mice than those immunized with LBNSE at 3 and 4 wpi as shown in Figure 2D. Moreover, significantly higher level of IgG1, which is associated with the stimulation of Th2-type immune response, was detected in mice immunized with LBNSE-IL15 than those immunized with LBNSE as early as 2 wpi and last till 5 wpi as shown in Figure 2E. In contrast, significantly higher level of IgG2a and IgG2b, which is stimulated during Th1-type immune response, were detected in mice immunized with LBNSE-IL15 than the mice immunized with LBNSE at 5 wpi and 4 wpi, respectively (Figure 2F, 2G). These data indicated that the expression of IL-15 by rRABV prefer to enhance the Th2-type immune response.
Furthermore, to investigate the protection against pathogenic RABV challenge, the immunized mice were challenged by IC route with 50 LD 50 of CVS-24 on 21 dpi and observed daily for 3 weeks. As shown in Figure 2H, mock immunized mice all died by 13 days post challenge, while 84% and 68% of mice immunized with LBNSE-IL15 or LBNSE were protected respectively, indicating that LBNSE-IL-15 could improve the survivorship of immunized mice after lethal viral challenge.
-
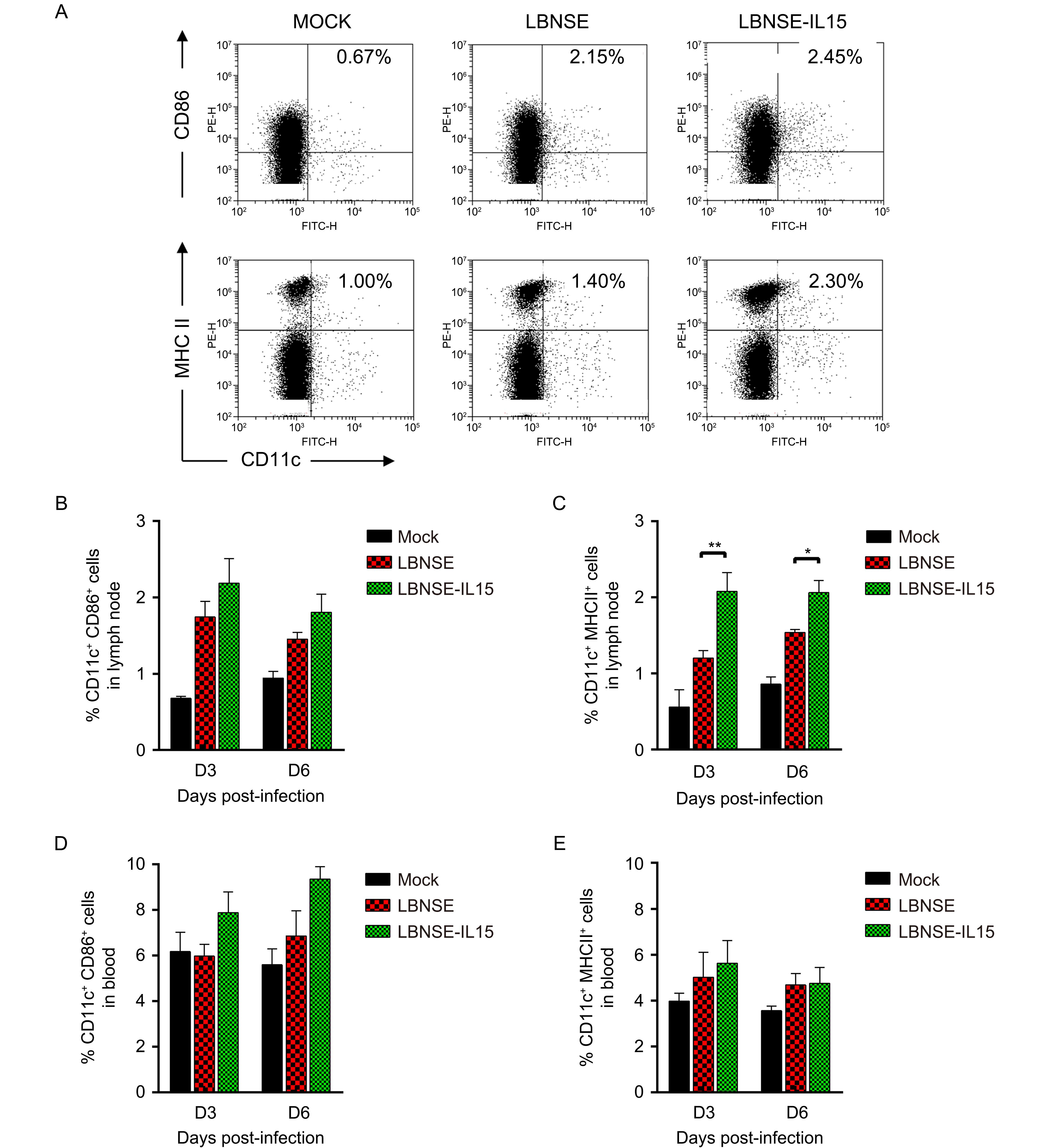
To investigate whether the maturation of DCs contribute to enhancement of VNA induction and better protection in mice immunization with LBNSE-IL15, mice were immunized with 106 FFU of LBNSE or LBNSE-IL15, or mock immunized with the same volume of DMEM as control; the draining lymph nodes and blood samples were collected at 3 and 6 dpi for detection of the maturation of DCs by flow cytometry. The representative flow cytometric plots of mature DCs (CD11c+CD86+ or CD11c+MHC II+) were as shown in Figure 3A respectively. For detection in lymph node samples, no significantly more maturation of DCs (CD11c+CD86+) were observed in lymph nodes of mice immunized with LBNSE-IL15 than those in LBNSE immunized mice as shown in Figure 3B; however, as shown in Figure 3C, significantly more mature DCs (CD11c+MHC II+) were detected in lymph nodes of mice immunized with LBNSE-IL15 than those in mice immunized with LBNSE both at 3 dpi and 6 dpi, indicating that expression of IL-15 by LBNSE-IL15 could enhance the maturation of DCs (CD11c+MHC II+) in immunized mice. No significant differences on the maturation of DCs were observed in peripheral blood between the mice immunized with LBNSE-IL15 and LBNSE either at 3 or 6 dpi as shown in Figures 3D, 3E.
-
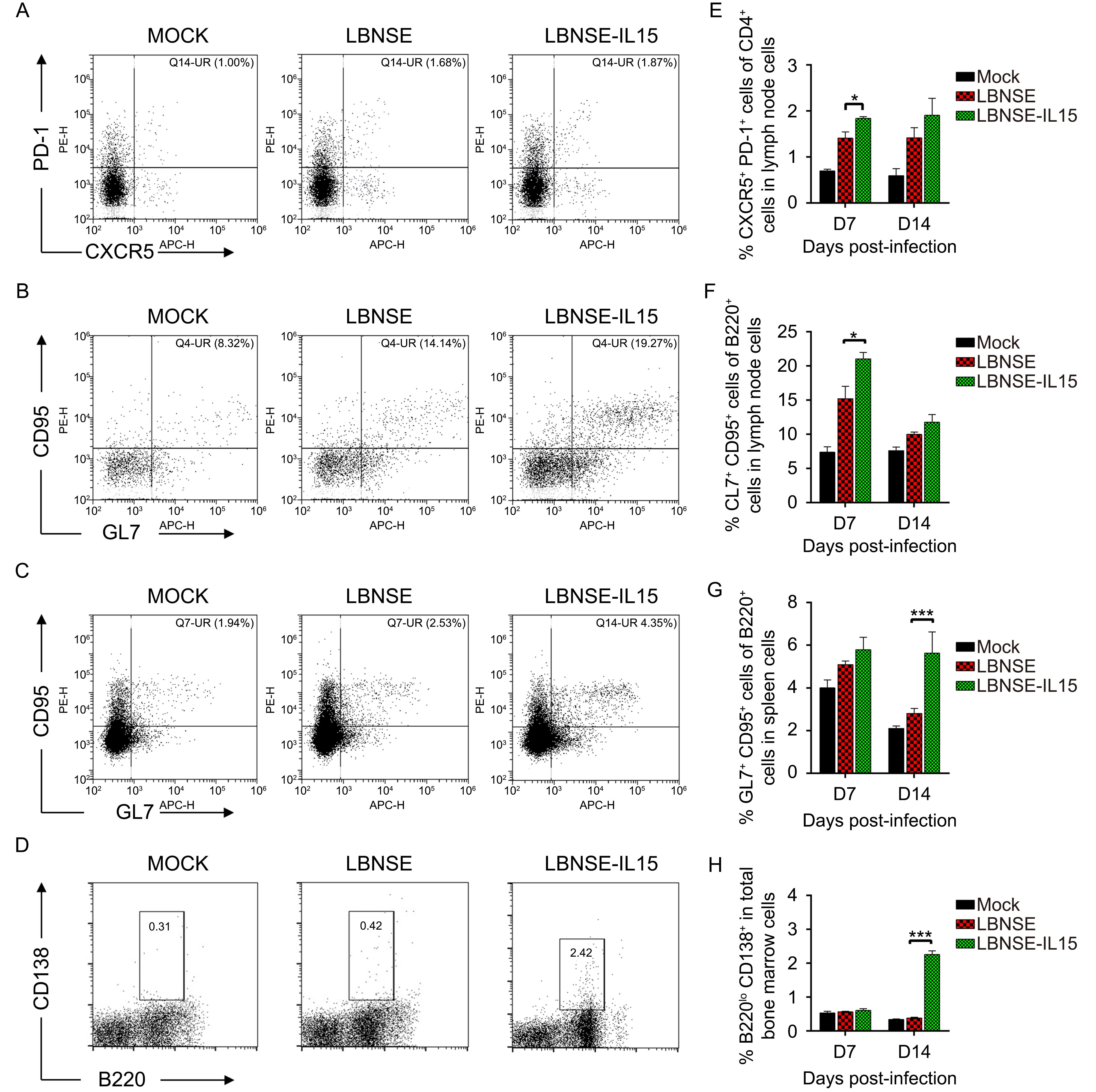
It was found in our study that LBNSE-IL15 could induce significantly higher level of VNA than LBNSE in immunized mice. Nevertheless, no significantly difference was observed on maturation of DCs in immunized mice. As is known that after antigen present to T cells by APC, CD4+ naïve T cells differentiate into several subtypes, and follicular helper T (T FH) cells are one of them, which are important for the germinal center (GC) formation and generating and selecting GC B cells with higher affinity for the pathogens. Hence, we next examined whether the generation of T FH and GC B cell were regulated during the induction of VNA. Mice were immunized with different rRABVs or mock immunized with DMEM, and the draining lymph nodes were collected for both at 7 and 14 dpi to detect the developments of T FH and GC B cells. The representative flow cytometric plots of T FH (CD4+CXCR5+PD-1+) and GC B (B220+CD95+GL7+) cells in lymph nodes were as shown in Figures 4A and 4B respectively. Significant more T FH cells and GC B cells were detected in lymph nodes of mice immunized with LBNSE-IL15 than those immunized with LBNSE at 7 dpi rather than 14 dpi (Figures 4E, 4F). In addition, spleens of immunized mice were also collected at 7 and 14 dpi to detect the generation of GC B cells. As shown in Figure 4G, significantly more GC B cells were observed in spleens of mice immunized with LBNSE-IL15 than those immunized with LBNSE at 14 dpi. These results suggest that expression of IL-15 by rRABV could enhance the generation of T FH cells and GC B cells after in immunized mice.
-
GC B cells can further differentiate into plasma cells, which then leave secondary lymphoid organs to reside primarily in bone marrow (Slifka et al., 1998; Hargreaves et al., 2001; Manz et al., 2005; Shapiro-Shelef and Calame, 2005; Tokoyoda et al., 2010). Therefore, to investigate whether LBNSE-IL15 could increase the generation of plasma cells, mice were vaccinated with each rRABVs or mock immunized with DMEM by i.m. route. Femur bone marrow was obtained from immunized mice and flow cytometry was carried out to detect the plasma cells (B220low & CD138 +). The representative flow cytometric plots of plasma cells in bone marrow were as shown in Figure 4D. As expected, significantly more plasma cells were detected in bone marrow of mice immunized with LBNSE-IL15 than those immunized with LBNSE at 14 dpi (Figure 4H).
Together, the data suggested that LBNSE-IL15 could promote the maturation of DCs and increase the generation of T FH cells, GC B cells, and plasma cells in immunized mice, by which enhancing the VNA induction level and providing a better protection.
-
Rabies is a preventable disease with prompt vaccination by inducing the virus-neutralizing antibody (VNA). DCs, the most efficient antigen-presenting cells (APCs), play a vital role during the process of antibody responses (Banchereau and Steinman, 1998). It was reported that only a small numbers of DCs were capable to directly stimulate the activated B cells proliferation, and promote the antibody production (Hargreaves et al., 2001). DCs can prime the CD4+ T cells, and the primed CD4+ T cells then interact with antigen-primed naïve B cells at the border between T cells and primary B cell follicles (Okada et al., 2005). These interactions of activated DCs with T and B cells are essential for the adaptive immune response induction (Brilot et al., 2008). Previous studies demonstrated that IL-15 could converts monocytes into DCs and then activate these DCs to initiate T cell responses (Mattei et al., 2001; Saikh et al., 2001; Anguille et al., 2013). Our previous studies indicated that recombinant attenuated RABV expressing chemokines or cytokines could enhance the immunogenicity through recruiting and/or activating DCs (Zhao et al., 2010; Zhou et al., 2013; Zhou et al., 2015; Wang et al., 2017). Consistent with these results, we found in this study that the expression of IL-15 could promote the maturation of DCs in lymph nodes of immunized mice.
After antigen presentation to T cells by APCs, CD4+ naïve T cells would differentiate into several subtypes, such as helper T type 1 (Th1), helper T type 2 (Th2), inducible regulatory T (iT Reg), interleukin-17-producing helper T (Th 17), or follicular helper T (T FH) cells (Crotty, 2014; Ueno et al., 2015). Among these subtypes, T FH cells play an important role in the formation of germinal center (GC), and regulate the GC B cells differentiation into plasma cells and memory B cells (Crotty, 2011; Crotty, 2014; Sage and Sharpe, 2016). In our previous study, a recombinant RABV expressing IL-21, another member of gamma chain receptor cytokine family along with IL-15, were constructed and investigated, and it was found that expression of IL-21 could increase the formation of T FH cells (Zhang et al., 2016b). Similarly, in this study, we also found that expression of IL-15 could enhance the generation of T FH cells in immunized mice compared with those immunized with parent virus LBNSE.
The GC response is a critical immune mechanism by which antibody affinity occurs, memory B cells develop, and long-lived plasma cells are produced. IL-15 can induce the germinal center B cells proliferation, differentiation, and Ig synthesis (Patidar et al., 2016). It was suggested that IL-15 helps in B cell proliferation through two pathways: IL-15-STAT5 and IL-15- SHC-Ras-Raf-ERK pathway; class-switching and antibody secretion also require IL-15 signaling (Litinskiy et al., 2002; Patidar et al., 2016). Due to the important role of IL-15 in the proliferation and Ig production by B cells, it can be a useful target where humoral response is compromised; several studies investigated the importance of IL-15 in B cell development, differentiation, and IgA level regulation (Hiroi et al., 2000). In case of HIV, IL-15 is a useful cytokine for enhancing the proliferation of anti-HIV B-cells and Ig production (Kacani et al., 1999). In our study, the expression of IL-15 by rRABV was found to generate significantly more GC B cells in lymph nodes and spleens of immunized mice, which further confirmed the role of IL-15 in promoting GC B cells formation.
As is known, GC B cells can further differentiate into plasma cells which lose the capacity to express chemokine C-X-C motif receptor 5 (CXCR5); plasma cells then leave the secondary lymphoid organs to reside primarily in bone marrow (Slifka et al., 1998; Hargreaves et al., 2001; Manz et al., 2005; Shapiro-Shelef and Calame, 2005; Tokoyoda et al., 2010). Plasma cells, the antibody-secreting cells, were investigated in this study, and it was found that significantly more plasma cells were detected in bone marrow of mice immunized with LBNSE-IL15 than those immunized with LBNSE at 14 dpi, which is consistent with the significantly higher level of VNA viters induced in LBNSE-IL15 immunized mice and higher protection after challenge.
In summary, our results indicate that expression of IL-15 by rRABV could enhance the immunogenicity by promoting the maturation of DCs, and increasing the generation of TFH cells, GC B cells, and plasma cells, indicating the potential of IL-15 as a promising adjuvant candidate for developing more efficient rabies vaccine.
-
This study was partially supported by the National Natural Science Foundation of China (31402176, 31372419, 31522057), the National Program on Key Research Project of China (2016YFD0500400), the Fundamental Research Funds for the Central Universities (2662016QD036, to MZ); the Ministry of Science and Technology of China (863 program, number 2011AA10A212), and the Ministry of Agriculture of China (special fund for Agro-scientific research in the Public Interest, 2013 03042, to ZFF).
-
The authors declare that they have no conflict of interest. Animal experiments in this study were approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2015-017).
-
ZFF, LZ, MZ conceived the experiments. TGC, YJZ, ZW, MML, JY, KLW performed the experiments. MC, LZ, MZ analyzed the data. TGC, YJZ, MZ wrote the manuscript and prepared the Figures. ZFF, LZ, MZ checked and finalized the manuscript. All authors read and approved the final manuscript.
Recombinant rabies virus expressing IL-15 enhances immunogenicity through promoting the activation of dendritic cells in mice
- Received Date: 19 June 2017
- Accepted Date: 09 August 2017
- Published Date: 29 August 2017
Abstract: Rabies remains a public health threat that kills approximately 59,000 people worldwide each year, most of which are from the developing countries of Africa and Asia where dog rabies are endemic. Therefore,developing an affordable and efficacious vaccine is crucial for rabies control in these countries.Interleukin (IL)-15,an immunoregulatory cytokine,is a pluripotent molecule with therapeutic potential,which targets many cell types and links the innate and adaptive immune system.In this study,IL-15 gene was cloned and inserted into the genome of a recombinant rabies virus (RABV) strain LBNSE (designated as LBNSE-IL15),and the effect of over-expression of IL-15 on the immunogenicity of RABV was investigated.It was found that mice vaccinated with LBNSEIL15 could induce significantly higher level of virus-neutralizing antibody (VNA) than those immunized with LBNSE,resulting in the higher protection after challenge.Further investigation was performed to find out the possible role of IL-15 plays in the process of antibody induction,and it was found that LBNSE-IL15 could enhance the maturation of dendritic cells (DCs) in immunized mice.Furthermore,the mice immunized with LBNSE-IL15 could promote the TFH cells differentiation and the generation of germinal center B cells and plasma cells.Together,these data indicated that IL-15 could be a potential adjuvant in enhancing the immunogenicity of RABV,contributing to the development of more-efficacious rabies vaccines.














 DownLoad:
DownLoad: