HTML
-
Chikungunya fever is a mosquito-borne disease caused by infection with chikungunya virus (CHIKV), an enveloped, single-stranded positive-sense RNA virus of the Alphavirus genus of the Togaviridae family. CHIKV is transmitted to people by Aedes mosquito bites, and its infection can result in various symptoms, including fever, rashes, and polyarthraμgia that may last for months (Garoff et al. 2004). There have been many CHIKV outbreaks in Africa, Asia, and Europe in the last decade, and a severe outbreak occurred in the Indian Ocean region, including India, in 2004 (Powers et al. 2000; Suhrbier et al. 2012). Thus, the risk of CHIKV reemerging and spreading worldwide by infected travelers is now a global health concern. Despite its importance, it is difficult to accurately identify CHIKV infection via clinical diagnosis because the clinical manifestations of CHIKV infection are similar to those of other mosquito-borne diseases, including those of various arboviruses, such as DENV, ZIKV, and JEV, which are classified into the Flavivirus genus of the Flaviviridae family (Roth et al. 2014; Wu et al. 2018).
Diagnosis of CHIKV infection is commonly based on two different detection methods: one based on viral RNA, and the other based on viral proteins. Detection of viral RNA by reverse transcription-polymerase chain reaction (RT-PCR) or viral antigen by anti-CHIKV mAbs is sensitive, but its use is limited by the short period of viremia during the early phase of CHIKV infection. On the other hand, diagnostic approaches capable of detection of antiCHIKV antibodies can be used after the acute phase of CHIKV infection and is a more accurate, complementary, and reliable method (Mardekian and Roberts 2015; Burdino et al. 2016; Johnson et al. 2016). The detection of anti-CHIKV antibodies or viral antigen relies on several techniques, including enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA), and lateral flow immunochromatographic assay (LFCA), and a key factor in these diagnostic approaches is a robust and accurate reaction between the viral antigen and the antibody. Although the several protein-based diagnostic tools are available to detect CHIKV infection, recent CHIKV diagnostic techniques are limited by low sensitivity (Mardekian and Roberts 2015; Burdino et al. 2016). Therefore, we ultimately need more sensitive and specific antibodies to improve CHIKV diagnosis.
CHIKV has two envelope glycoproteins, E1 and E2, that cover the viral surface with spike structures and mediate viral entry into its host cells (Strauss and Strauss 1994). E2 is responsible for cell attachment, while E1 controls membrane fusion during viral infection. The extracellular part of the E2 protein contains domains for receptor binding and is immunogenic (Strauss and Strauss 1994; Smith et al. 1995; Jin et al. 2015; Weger-Lucarelli et al. 2015). Thus, the most abundant antibodies triggered by CHIKV infection appear to target CHIKV-E2 protein (Kam et al. 2012a, b). Thus, the CHIKV-E2 protein is an excellent target for the development of anti-CHIKV-E2 monoclonal antibodies (mAbs) with high binding affinity and specificity to improve the CHIKV diagnostic techniques.
Here, we have generated anti-CHIKV-E2 mAbs via hybridoma systems and investigated their sensitivity and specificity by comparing them to commercial mAbs. Our newly generated anti-CHIKV-E2 mAbs, 19-1 and 21-1, had robust binding affinities to CHIKV-E2 protein and inactivated CHIKV. In particular, the 19-1 mAb significantly bound to CHIKV and barely recognized other mosquito-transmitted viruses, including DENV, ZIKV, and JEV.
-
Six-week-old female BALB/c mice were purchased from Orient Bio (Gyeonggi-do, Republic of Korea) and were kept in a specific pathogen-free facility in the Korea Research Institute of Bioscience and Biotechnology (KRIBB). CHIKV-E2 was purchased from Sino Biological (Beijing, China). Mice were immunized 3 times with 10 μg of CHIKV-E2 mixed with TiterMax Gold adjuvant (SigmaAldrich, St. Louis, MO, USA) by footpad injection at 2-week intervals. Two weeks after each immunization, serum samples were collected from the immunized mice. Two weeks after the last immunization, cells were isolated from the popliteal lymph nodes and used to prepare B cell hybridomas that produce anti-CHIKV-E2 antibodies. The fusion with myeloma FO cells (ATCC CRL 1646) was performed as previously described (Heo et al. 2010). The binding affinities of the antibodies produced by these hybridomas were measured by ELISA. The mAbs were purified using protein G columns (GE Healthcare, Uppsals, Sweden). The isotype of each mAb was determined with an immunoglobulin isotyping kit (Roche Diagnostics, Mannheim, Germany).
-
ZIKV (MR766 strain; ATCC VR84) was propagated and maintained in Vero E6 cells. JEV (SA14-14-2 strain; Korea Vaccine Corp., Gyeonggi-do, Republic of Korea) was propagated and maintained in BHK-21 cells. The Vero E6 (ATCC CRL1586) and BHK-21 (ATCC CCL10) cells were maintained in Eagle's minimum essential medium (HyClone Laboratories, Inc., UT, USA) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Inc.). All of the cell lines were cultured at 37 ℃ with 5% CO2. The cells were seeded in T75 flasks at 60% confluence and cultured for 16 h. The cells were infected with 5 mL of virus stock suspension diluted 1:25 with fresh culture medium. The suspensions were replaced for virus propagation, and the culture supernatants were harvested after 3–5 days to obtain viruses. The culture supernatants were filtered and precipitated for 12 h at 4 ℃ in PBS. The viral precipitates were pelleted by centrifugation (14, 000×g, 1 h, 4 ℃), resuspended in 300 μL sterile PBS, and stored at - 80 ℃. The infectivity titers were estimated based on the number of plaque forming units (PFUs) observed in plaque assays. The viruses were inactivated by incubation for 30 min at 65 ℃ and then used for the experiments. Inactivated viral lysates of CHIKV and DENV types 1–4 were obtained from ZeptoMetrix Corporation (ZeptoMetrix Corp., Franklin, MA, USA).
-
MaxiSorp 96-well plates (Thermo Scientific, Roskilde, Denmark) were coated with CHIKV-E2 (5 μg/mL) or inactivated CHIKV lysates (90 μg/mL) in PBS or 1% human serum (Sigma-Aldrich) diluted PBS for 16 h at 4 ℃, blocked with 5% skim milk in PBS for 1 h at 37 ℃, and washed with 0.05% Tween-20 in PBS (PBST). The plates were then incubated with 1:50, 000-diluted serum from CHIKV-E2 immunized mice, the culture supernatants from the primary clones, or purified mAbs (10 μg/mL) for 2 h at 37 ℃, followed by further incubation with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1:5000) (Cell Signaling Technologies, Danvers, MA, USA) for 1 h at 37 ℃. After the plates were washed with PBST, the reactions were developed with the chromogenic tetramethylbenzidine substrate (BD Biosciences, San Diego, CA, USA) and terminated with 2 N H2SO4. Optical density (OD) was measured at 450 nm using a Versamax microplate reader (Molecular Devices, San Francisco, CA, USA). The commercially available anti-CHIKV-E2 mAbs, 16A12 and Chk265, were purchased from The Native Antigen (Oxford, United Kingdom) and Absolute Antibody (Oxford, United Kingdom), respectively.
In the cross-reactivity experiment, MaxiSorp 96-well plates were coated with 100 μL of inactivated viral lysates containing CHIKV (10 μg/mL), ZIKV (1.7 × 105 PFU/mL), JEV (1.7 × 105 PFU/mL) or DENV types 1–4 (10 μg/mL) in carbonate–bicarbonate buffer, pH 9.6, for 16 h at 4 ℃. In separate experiments, ELISA plates were coated with serially diluted inactivated viral lysates. The plates were then incubated with 5% skim milk in PBS for 2 h at RT, washed with 0.025% Tween-20 in PBS, and then further incubated with our newly generated anti-CHIKV-E2 mAbs (1 μg/mL) for 1 h 30 min at 37 ℃, followed by further incubation with HRP-conjugated anti-mouse IgG (1:5000) (Thermo Fisher, Rockford, IL, USA) for 1 h at 37 ℃. The plates were washed and developed with the chromogenic tetramethylbenzidine substrate (Thermo Fisher). The reactions were terminated with 2 N H2SO4, and the absorbance was measured at 450 nm with a Versamax microplate reader (Molecular Devices).
-
CHIKV-E2 (1 μg) or inactivated CHIKV lysate (3 μg) were incubated in reducing sample buffer for 5 min at 37 ℃ and heated for 5 min at 95 ℃. The samples were separated by 12% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred onto polyvinylidene fluoride membranes (Bio-Rad). After blocking with 5% skim milk in TBS containing 0.05% Tween-20, the membranes were incubated with the anti-CHIKV E2 mAbs (2 μg/mL) or the commercial mAbs for 12 h at 4 ℃. Next, the membranes were washed and then further incubated with HRP-conjugated anti-mouse IgG (1:5000) (Cell Signaling Technologies). The immunoreactive bands were developed with Amersham ECL Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, United Kingdom) and visualized using an Azure C300 Western blot imager (Azure Biosystems, Dublin, CA, USA).
Mouse Immunization and Hybridoma Preparation
Viruses
ELISA
Immunoblotting
-
To generate anti-CHIKV-E2 mAb-secreting hybridomas, the recombinant CHIKV-E2 protein was used as an immunogen. As shown in Fig. 1A, a coomassie brilliant blue dye-stained SDS-PAGE gel showed that the size of the recombinant CHIKV-E2 protein was approximately 40 kDa. Immunoblotting also confirmed that the recombinant CHIKV-E2 protein was detected by the commercial anti-CHIKV-E2 mAbs. Thus, mice were immunized 3 times with recombinant CHIKV-E2 protein mixed with adjuvants in their footpads at 2-week intervals. Two weeks after each immunization, serum samples were obtained from the immunized mice and assessed by ELISA to monitor the production of anti-CHIKV-E2 Abs. The level of the anti-CHIKV-E2 Abs in the serum was four-fold higher after the third immunization compared with that after the first immunization (Fig. 1B), indicating that repeated immunization with recombinant CHIKV-E2 protein drastically induces production of anti-CHIKV-E2 Abs. Next, lymphocytes were obtained from the popliteal lymph nodes of the mice 2 weeks after the last immunization, and the lymphocytes were then fused with FO myeloma cells to generate hybridomas that secrete anti-CHIKV-E2 mAbs. ELISAs were performed using the culture supernatants from the hybridomas to confirm the generation of Ab-secreting hybridomas. Among 58 primary clones, 10 clones had higher OD values to the CHIKV-E2 protein compared with the values obtained using BSA as a negative control (Fig. 1C). In particular, the 4 primary clones called 19, 21, 22, and 26 showed OD values above 2.5, suggesting that these hybridomas effectively produce antiCHIKV-E2 Abs. Thus, we performed subcloning and selected 4 monoclones, designated 19-1, 21-1, 22-2, and 26-4, for further experiments.
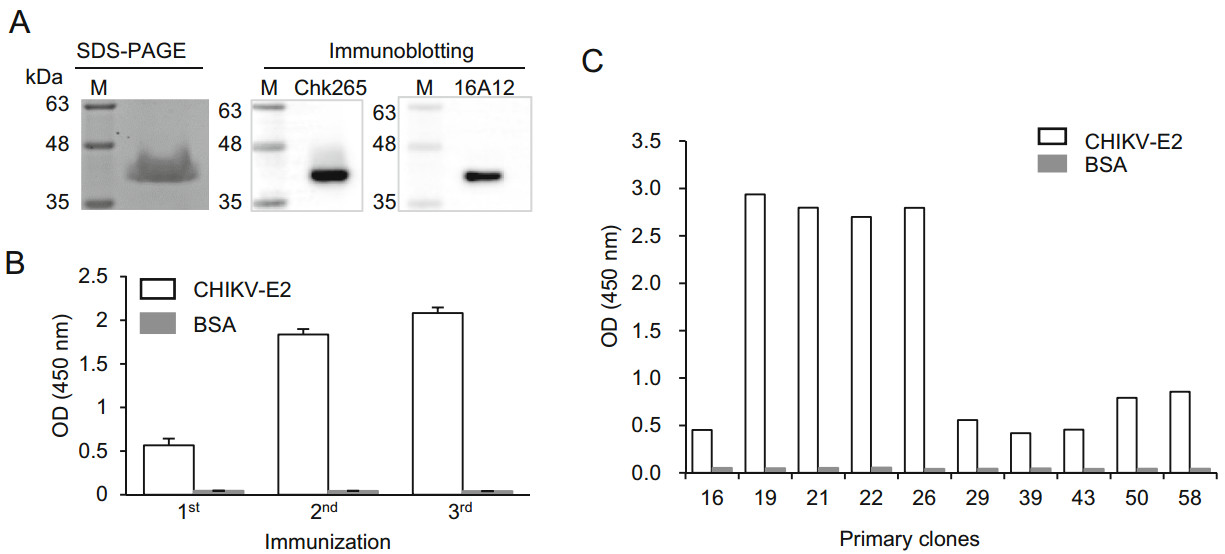
Figure 1. Production of anti-CHIKV-E2 mAb-secreting hybridomas. A CHIKV-E2 protein was separated by 12% SDS-PAGE and visualized by Coomassie blue staining. For immunoblotting, the protein was transferred from the gel onto a PVDF membrane and probed with commercial mAbs (Chk265 or 16A12), followed by incubation with HRP-conjugated anti-mouse IgG. Lane M indicates a protein marker (kDa). B, C BALB/c mice were immunized 3 times with CHIKV-E2 protein via footpad injection at 2-week intervals. Serum samples were collected 2 weeks after each immunization (B), and the culture supernatant was harvested from the hybridomas (C). Binding affinities against CHIKV-E2 protein were determined by ELISA. Corrected OD values are shown.
-
We firstly investigated whether the newly generated mAbs recognize a linear or conformational epitopes of the CHIKV-E2 protein. As shown in Fig. 2A, immunoblotting showed that all of the anti-CHIKV-E2 mAbs detected CHIKV-E2 protein, suggesting that the mAbs can recognize linear epitopes in the CHIKV-E2 protein (Fig. 2A). The isotypes of the mAbs were determined using antibody isotyping kits (Table 1). The 19-1 and 22-2 mAbs were IgG2b kappa-chain isotypes, while 21-1 and 26-4 were IgG1 kappa-chain isotypes. To compare the binding affinities of the anti-CHIKV-E2 mAbs, we performed ELISAs using various amounts of either the mAbs or CHIKV-E2. Two commercially available anti-CHIKV-E2 mAbs, 16A12 and Chk265, were used for comparison. When various amounts of the generated mAbs were applied on CHIKV-E2 protein-coated plates, the 21-1 mAb showed a higher OD value than those of both the commercial mAbs, and the 19-1 mAb had similar OD values with those of Chk265 (Fig. 2B). Two other mAbs, 22-2 and 26-4, showed lower OD values compared with those of both commercial mAbs. Next, we performed ELISAs using the generated mAbs on the plates coated with serially diluted CHIKV-E2 protein, and all of the mAbs exhibited higher OD values compared with those obtained using the commercial mAbs (Fig. 2C). Notably, the 19-1 mAb showed the highest OD value on the plates coated with CHIKV-E2 protein, ranging from 1.2 to 312.5 ng/mL. As a spiking experiment, we performed ELISA using CHIKVE2 protein in PBS containing 1% human serum. The mAbs, 19-1 and 21-1, showed higher reactivity to CHIKV-E2 protein in the human serum than other commercial mAbs (Fig. 2D). These results indicate that the 19-1 and 21-1 mAbs have higher binding affinities to CHIKV-E2 protein compared with those of the commercial mAbs and recognize linear epitopes in the CHIKV-E2 protein.
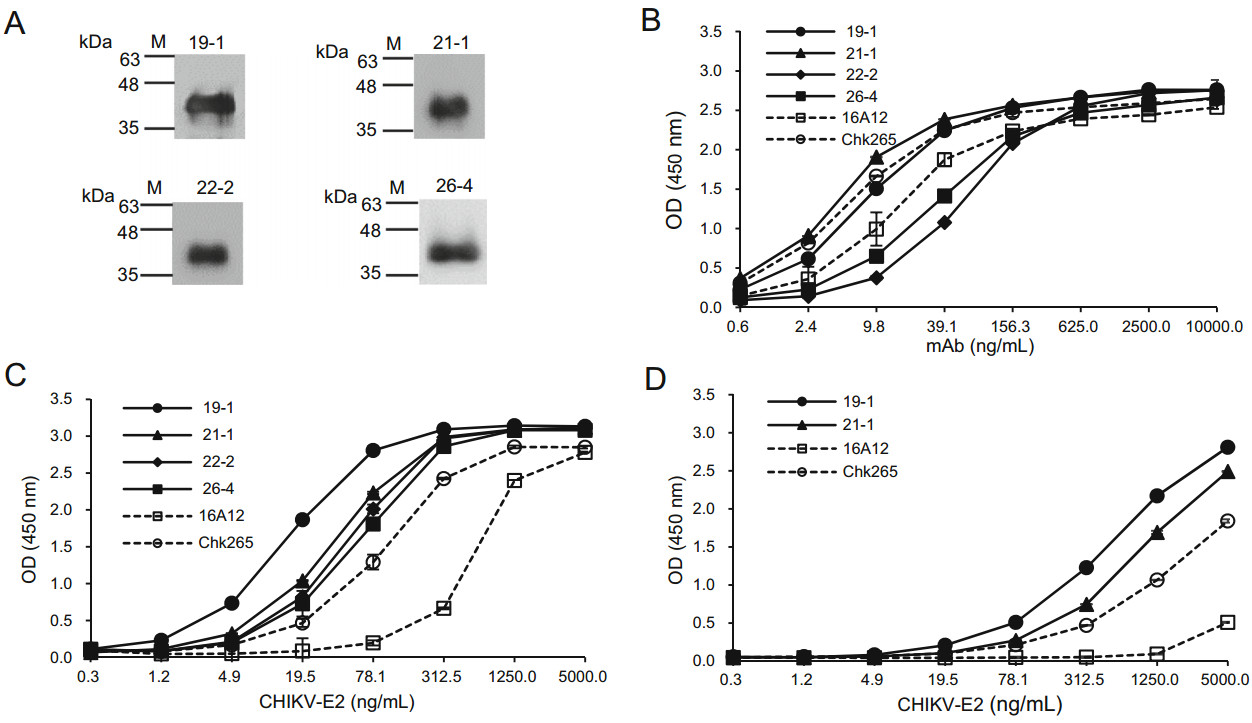
Figure 2. Comparison of the binding affinities of the anti-CHIKV-E2 mAbs to the CHIKV-E2 protein. A CHIKV-E2 protein was separated by 12% SDS-PAGE and transferred onto a PVDF membrane. The membranes were probed with the anti-CHIKV-E2 mAbs, followed by incubation with HRP-conjugated anti-mouse IgG. Lane M indicates a protein marker (kDa). B ELISA plates were coated with CHIKV-E2 protein (5 μg/mL), The plates were blocked with 5% skim milk in PBS and treated with serially diluted anti-CHIKV-E2 mAbs, followed by incubation with HRP-conjugated anti-mouse IgG. C CHIKV-E2 protein was coated onto ELISA plates in a dose-dependent manner, blocked, and treated with the anti-CHIKV-E2 mAbs (10 μg/mL), followed by incubation with HRP-conjugated anti-mouse IgG. D As a spiking experiment, ELISA plates were coated with CHIKV-E2 protein (5 μg/mL) in PBS containing 1% human serum, blocked, and treated with the anti-CHIKV-E2 mAbs (10 μg/mL), followed by incubation with HRP-conjugated anti-mouse IgG. Commercial antiCHIKV-E2 mAbs (Chk265 and 16A12) were used for comparison. Corrected OD values are shown.
Clone Isotype Light chain EC50 against CHIKV (ng/mL) 19-1 IgG2b κ 13.86 21-1 IgG1 κ 20.45 22-2 IgG2b κ – 26-4 IgG1 κ – 16A12 IgG1 – 24.58 Chk265 IgG1 λ 22.75 Table 1. Isotypes and EC50 values of the anti-CHIKV-E2 mAbs.
-
To investigate whether the newly generated mAbs recognize CHIKV or other mosquito-transmitted viruses, we performed ELISAs using the 19-1 and 21-1 mAbs that bound tightly to the CHIKV-E2 protein. First, inactivated CHIKV was serially diluted and coated onto ELISA plates. When consistent amounts of the anti-CHIKV-E2 mAbs were applied to the plates, the ELISA results showed that the 19-1 mAb had the highest OD value compared with those of the 21-1 mAb and the commercial mAbs (Fig. 3A). The 21-1 mAb had OD values similar to those of the commercial Chk265 mAb. In contrast, the commercial 16A12 mAb showed the lowest OD value. Next, we performed ELISAs using various amounts of the mAbs on the inactivated CHIKV-coated plates. Consistently, the 19-1 mAb had the highest OD value compared with those of the other mAbs, while the 21-1 mAb showed similar OD values with the commercial Chk265 mAb (Fig. 3B). Next, we quantitatively analyzed the binding affinities of the mAbs by calculating the half-maximal effective concentration (EC50). As shown in Table 1, the EC50 of the 19-1 mAb (13.86 ng/mL) was the lowest compared with those of the other mAbs (20.45 ng/mL for the 21-1 mAb, 22.75 ng/mL for the Chk265 mAb, and 24.58 ng/mL for the 16A12 mAb). Additionally, immunoblotting showed that the 19-1 and 21-1 mAbs detected inactivated CHIKV at a bind size of approximately 50 kDa, which matched the predicted molecular mass of the CHIKV-E2 (Warter et al. 2011). For the commercial mAbs, Chk265 recognized the viral CHIKV-E2 protein, while 16A12 barely detected the protein (Fig. 3C). These results indicate that the 19-1 mAb efficiently binds to CHIKV.
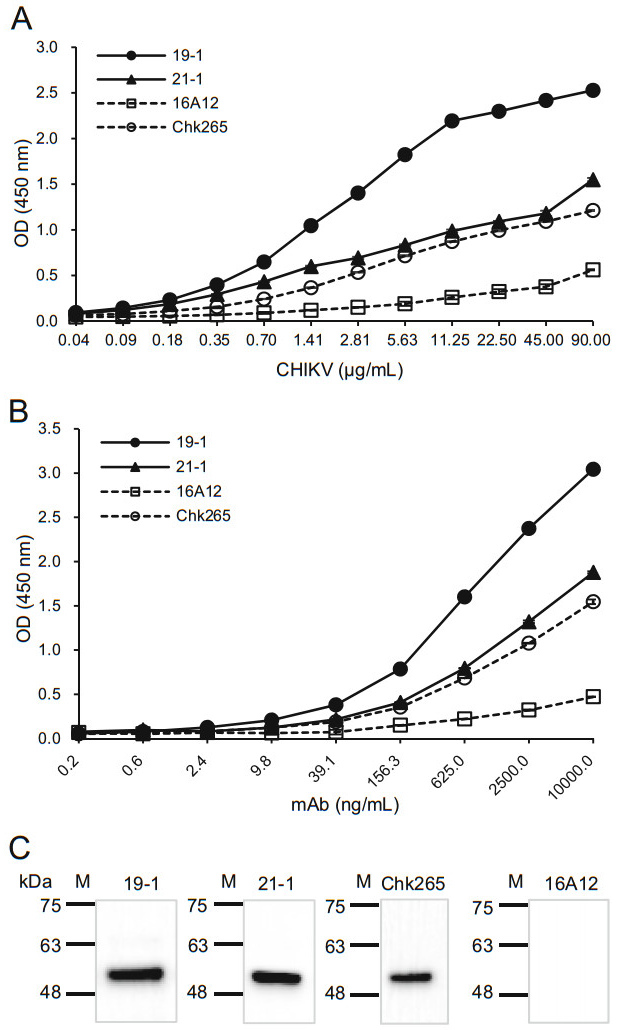
Figure 3. Binding affinities of the anti-CHIKV-E2 mAbs against inactivated CHIKV. A Serially diluted inactivated CHIKV was coated onto ELISA plates. The plates were blocked with 5% skim milk in PBS, incubated with the anti-CHIKV-E2 mAbs (10 μg/mL), followed by incubation with HRP-conjugated anti-mouse IgG. B ELISA plates were coated with the inactivated CHIKV (90 μg/mL), blocked, and incubated with serially diluted anti-CHIKV-E2 mAbs, followed by incubation with HRP-conjugated anti-mouse IgG. Corrected OD values are shown. C Inactivated CHIKV proteins were separated by 12% SDS-PAGE and transferred onto PVDF membranes. The membranes were probed with the anti-CHIKV-E2 mAbs, followed by incubation with HRP-conjugated anti-mouse IgG. Commercially available anti-CHIKV-E2 mAbs (Chk265 and 16A12) were used for comparison. Lane M indicates a protein marker (kDa).
Because CHIKV is one of the known arboviruses that cause mosquito-borne diseases, we lastly investigated whether our newly generated mAbs can discriminate CHIKV from other mosquito-transmitted arboviruses, including ZIKV, JEV, and DENV types 1–4. ELISA plates were coated with inactivated arboviruses, and the binding affinities were compared using the anti-CHIKV-E2 mAbs followed by incubation with HRP-conjugated anti-mouse IgG. Chk265 was used for comparison, and PBS was used as a negative control. The ELISAs showed that the 19-1 mAb showed significantly higher OD value only against CHIKV (Fig. 4A). In contrast, the 21-1 mAb showed significant increases in the OD value against DENV types 1–4, as well as CHIKV, compared with the value obtained with the PBS control (Fig. 4B). The commercial Chk265 mAb showed significantly high OD value against CHIKV and DENV types 2–4. To further confirm the lack of binding to other arboviruses of the 19-1 and 21-1 mAbs, we performed ELISAs using the plates coated with serially diluted inactivated arboviruses. As expected, the 19-1 and 21-1 mAbs showed higher OD values against the inactivated CHIKV and barely bound to various amounts of the inactivated ZIKV, JEV, and DENV types 1–4 (Fig. 4D–J). These results indicate that the 19-1 mAb binds significantly to CHIKV but not to the other arboviruses.
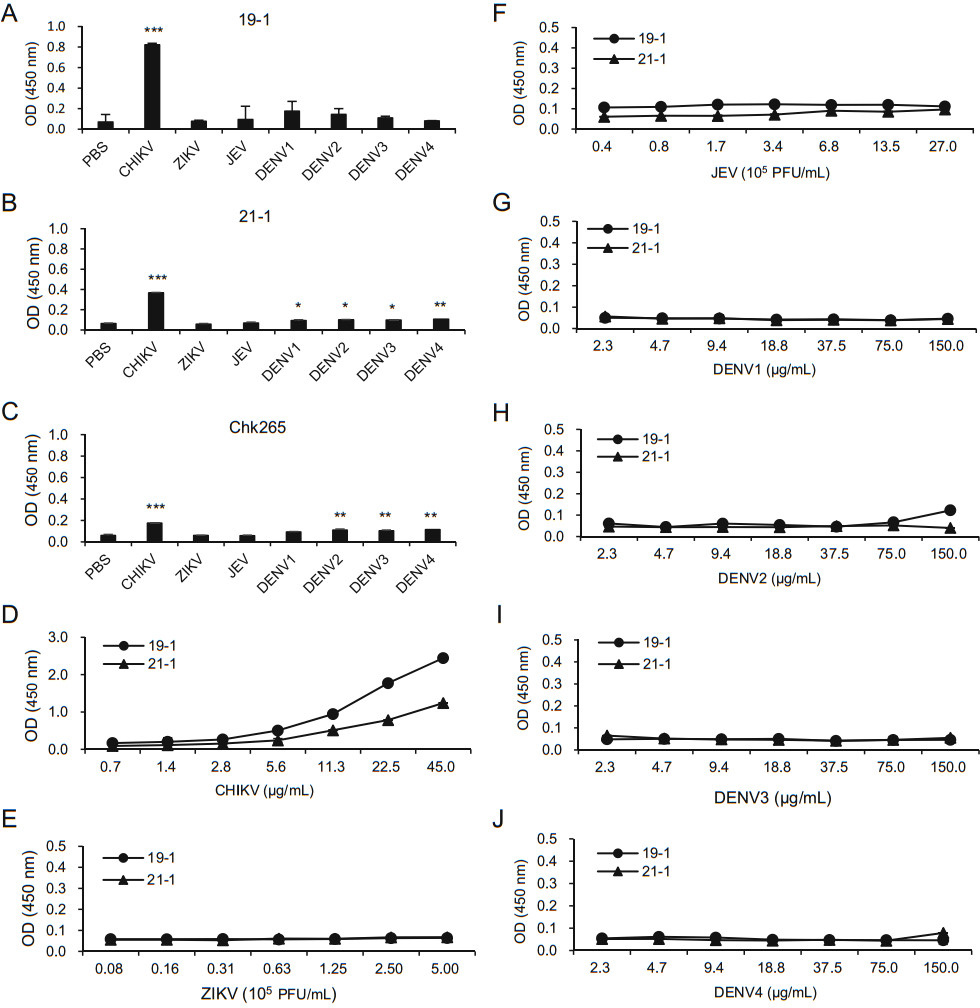
Figure 4. Specificity of the anti-CHIKV E2 mAbs. Inactivated arboviruses (CHIKV, ZIKV, JEV, and DENV types 1–4) were coated onto ELISA plates. After blocking with 5% skim milk in PBS, the plates were incubated with A the 19-1 mAb (1 μg/mL), B the 21-1 mAb (1 μg/mL), and C the Chk265 mAb (1 μg/mL), followed by incubation with HRP-conjugated anti-mouse IgG. D–J ELISA plates were coated with serially diluted viral lysates such as inactivated CHIKV (D), ZIKV (E), JEV (F), and DENV type 1 (G), DENV type 2 (H), DENV type 3 (I) and DENV type 4 (J). After blocking with 5% skim milk in PBS, the plates were incubated with the 19-1 and 21-1 mAb (1 μg/mL), followed by incubation with HRP-conjugated anti-mouse IgG. Corrected OD values are shown. Significant differences were analyzed by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
Production of Anti-CHIKV-E2 mAbs
Comparison of the Binding Affinities and Characterization of the Anti-CHIKV-E2 mAbs
Sensitivity and Specificity of the Anti-CHIKV-E2 mAbs to Viruses
-
To prevent and control CHIKV infection, it is important to develop accurate and rapid diagnostic approaches that can identify infectious CHIKV virus at the early stage of infection. Thus, it is essential to generate anti-CHIKV mAbs with high sensitivity and specificity that can recognize small amounts of CHIKV and accurately distinguish CHIKV from other mosquito-transmitted viruses. In this study, we generated new anti-CHIKV-E2 mAbs and found that the 19-1 and 21-1 mAbs more effectively bound to the CHIKV-E2 protein than two commercially available antiCHIKV-E2 mAbs did. Importantly, our 19-1 mAb significantly bound to inactivated CHIKV and had no cross-reactivities against other mosquito-transmitted viruses, including ZIKV, JEV, and DENV.
Our results showed that the newly generated 19-1 and 21-1 anti-CHIKV-E2 mAbs efficiently bound to the CHIKV-E2 protein in PBS and human serum. Notably, the 19-1 mAb showed higher binding affinities to various amounts of CHIKV-E2 protein (1.2–312.5 ng/mL in PBS or 19.5–5000 ng/mL in 1% human serum) compared with those of the other mAbs. More importantly, the 19-1 mAb bound most strongly to inactivated CHIKV (0.7–90 μg/mL). However, the 21-1 mAb had a lower binding affinity to inactivated CHIKV compared with that of the 19-1 mAb, even though the 19-1 and 21-1 mAbs had similar binding affinities to CHIKV-E2 protein. Several studies have revealed that domain A of E2 is located at the distal end and is mainly exposed on the viral surface, while domains B and C are located in the center of E2 and are close to the viral membranes, respectively (Voss et al. 2010). The surface structure of the CHIKV virion is covered with 80 trimeric spikes, and each single spike consists of three E1/E2 heterodimers (van Duijl-Richter et al. 2015). Thus, we speculate that the 19-1 mAb may bind to an epitope exposed on the viral surface, whereas the 21-1 and may recognize an epitope inside the viral surface or a region hidden via the dimerization of E1 and E2. Further study is needed to elucidate high binding affinities of the 19-1 mAb compared to other antibodies, because computational structure analysis is suitable for predicting the binding affinity of the antibody against CHIKV-E2 antigen based on the amino acid sequence (Sela-Culang et al. 2013).
Our results revealed that the 19-1 mAb barely recognized other mosquito-transmitted viruses, including ZIKV, DENV, and JEV. In contrast, the 21-1 mAb had significantly higher binding affinities to CHIKV as well as to DENV types 1–4 than it showed with the PBS control. Currently, clinical diagnosis is difficult during the early stage of CHIKV infection because the clinical symptoms are similar among the mosquito-transmitted viruses (Chen and Wilson 2010; Hoarau et al. 2010). Thus, molecular diagnostic approaches are important to accurately diagnose the infection, as CHIKV belongs to the genus Alphavirus of the Togaviridae family, while DENV, JEV, and ZIKV belong to the genus Flavivirus of the Flaviviridae family (Garoff et al. 2004; Bhatt et al. 2013; Fauci and Morens 2016). The CHIKV genome is also quite different from the genome of the flaviviruses, and the nucleotide sequences of structural protein between DENV and CHIKV show no similarity (Bhandarkar and Singhal 2011). Therefore, our newly generated 19-1 anti-CHIKV-E2 mAb could be better for distinguishing CHIKV from other mosquito-transmitted viruses. Moreover, CHIKV is closely related to other Alphaviruses. Regarding the potential cross-reactivity of the CHIKV mAb with the other alphaviruses, it is reported that CHIKV-E2 protein has more than 50% amino acid sequence identity to the other Alphavirus (e.g., 83.0% to O'nyong'nyong virus, 57.6% to Semliki Forest virus, 56.6% to Ross River virus, and 56.2% to Mayaro virus) (Fox et al. 2015). Due to difficulties in obtaining other Alphaviruses, the present study did not examine the crossreactivity of the CHIKV mAbs with other alphaviruses. Thus, further study is needed to investigate whether our anti-CHIKV mAbs can have cross-reactivity against other Alphaviruses.
Protein-based CHIKV diagnostic kits have been used and are based on several methods, including ELISA, IFA, and ICA (Brehin et al. 2008; Shukla et al. 2009; Mardekian and Roberts 2015; Okabayashi et al. 2015; Fumagalli et al. 2018; Jain et al. 2018). Thus, the CHIKV mAb can be useful for the direct IFA to detect viral antigen at early phase of CHIKV infection. In addition, the CHIKV mAb can be adjustable to sandwich ELISA as a solid-phasebound antibody or LFCA as probe-conjugated antibody responsible for detecting the complex of antigen and antibody (analyte) to detect anti-CHIKV antibodies in the plasma of the patients after the acute phase of CHIKV infection. Currently, newly developed materials (e.g., nanoparticles and fluorescent dyes for antibody conjugation) have been adapted for use in various diagnostic methods to improve sensitivity. Further studies may be needed to determine the best approaches for optimizing the antibody activity depending on the various diagnostic methods. Although it is important to validate the sensitivity and specificity of CHIKV mAb using clinical isolates and human plasma samples, we are not able to obtain clinical samples of CHIKV-infected patients due to a few cases of CHIKV infection in Korea. Also, because CHIKV is designated as a communicable disease group Ⅳ in Korea, it is difficult to obtain clinical samples of CHIKV-infected patients until now. Thus, further study is need to validate our newly generated anti-CHIKV mAbs using samples from CHIKV-infected patients.
In conclusion, we report here the production of new anti-CHIKV-E2 mAbs that can be used in protein-based CHIKV diagnostic methods. Our anti-CHIKV-E2 mAbs, particularly the 19-1 mAb, have improved sensitivity against CHIKV compared with the sensitivities of two commercial mAbs and exhibited sufficient specificity to distinguish CHIKV from other mosquito-transmitted viruses. The risk of mosquito-borne diseases is a threatening global public health issue, and the production of improved anti-CHIKV antibodies with high sensitivity and specificity is crucial for the development of rapid and accurate diagnostic approaches. Therefore, the 19-1 mAb might be useful for the development new CHIKV diagnostic approaches that help prevent the spread of CHIKV.
-
This work was supported by Grants from the R & D Convergence Program of National Research Council of Science & Technology (No. CAP-16-02-KIST) and the National Research Foundation of Korea (No. NRF-2016M3A9B6918584).
-
HP designed the experiments; JK performed the experiments; SK, HL, and YK contributed to analyze crossreactivities of anti-CHIKV-E2 antibodies to arboviruses; JK and JY analyzed the experiments and drafted the manuscript; HP supervised the experiments, analyzed results, and wrote the manuscript. All authors approved the final manuscript.
-
The authors declare that they have no competing interests.
-
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Research Institute of Bioscience and Biotechnology (KRIBB) and performed according to the Guidelines for Animal Experiments of the KRIBB.














 DownLoad:
DownLoad: