HTML
-
Emerging and re-emerging viral infections pose severe challenges for diagnosis, treatment, and public health surveillance. Early etiological diagnosis is, therefore, very important for the control of sudden viral infections, and requires antibodies with both high sensitivity and high specificity. Monoclonal antibodies are widely used in clinical diagnosis and therapy owing to their specificity and high affinity. Monoclonal antibody preparation mainly involves hybridomas (Kohler and Milstein 1975), phage display (Smith 1985; Hoogenboom et al. 1991), and B cell sorting (Wrammert et al. 2008), among other techniques. However, these technologies do have limitations, such as a long and arduous cycle, complicated operation, and high expenses.
DT40, a chicken lymphoma cell line, produces IgM-type antibodies and undergoes gene conversion and somatic mutations in the variable region of immunoglobulin (IgV) gene during culture. The activation-induced cytidine deaminase (AID) gene is responsible for hyperconversion (Arakawa et al. 2002; Harris et al. 2002) and somatic hypermutation (Martin et al. 2002; Yoshikawa et al. 2002). The constitutive expression of AID in DT40 cells leads to the hypermutation of IgV, increases the diversity of IgMs, and makes DT40 cells a natural antibody library for monoclonal antibody screening (Cumbers et al. 2002; Seo et al. 2005). Furthermore, the fast growth of DT40 cells enables rapid antibody acquisition compared to that in hybridoma and phage display systems (Seo et al. 2006). Therefore, the DT40 antibody library is suitable for rapid generation of diagnostic antibodies for emerging viral infections.
After the selection of antigen-specific clones, expression of AID in the isolated clones must be inhibited to stop the hypermutation. To this end, Kanayama et al., introduced a Cre-loxP system-based switching device into the endogenous AID gene of DT40 cells (Kanayama et al. 2005). However, the repeated switching of AID expression, using this approach, required sorting or selection with puromycin, in addition to 4-hydroxytamoxifen treatment. In the present study, we generated a DT40 cell line with inducible AID expression, the latter being controlled in a simpler and more efficient manner. Using an antibody library generated from this cell line, we successfully obtained monoclonal antibodies against the NS1 protein of Zika virus.
-
DT40 cells, obtained from American Type Culture Collection (ATCC, USA), were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, 5% chicken serum, 2 mmol/L L-glutamine, and 0.05 mmol/L 2-mercaptoethanol. DT40 cells were treated with 0.625 ng/mL of trichostatin A (TSA) for 8 weeks to induce IgM diversification (Seo et al. 2006). All cell culture reagents were purchased from Thermo Fisher Scientific (USA). DT40 cells were transfected using the Neon Transfection System (Thermo Fisher Scientific) with 3 pulses at 1350 V and 10-ms pulse width.
-
First, the AID gene was knocked out in DT40 cells using the CRISPR/Cas9 genome editing system. A guide RNA was designed targeting the 2nd exon of AID gene, and the corresponding DNA oligonucleotides were ligated into the GeneArt CRISPR Nuclease Vector with an OFP reporter (Thermo Fisher Scientific). OFP-positive clones were selected from the vector-transfected DT40 cells using flow cytometry and AID levels were analyzed using Western blotting. AID knockout was further confirmed using DNA sequencing.
Thereafter, the Tet-Off expression system (Takara, Japan) was introduced to achieve the inducible expression of AID. The AID-deficient DT40 cells (DT40-dAID) were stably transfected with the pTet-Off vector (Takara), which expressed the Tet-responsive transactivator (TetR), followed by transfection with the pTight/AID/P2A/EGFP expression cassette. The transfected cells were treated with Zeocin (300 μg/mL) for 1 week and EGFP-positive cells were selected by flow cytometry. The EGFP-positive cell suspension was diluted to contain 30 cells in 10 mL culture medium and plated in 96-well plates for single clone formation. A cell clone (DT40-H7) was isolated and used in subsequent studies. AID expression was silenced in DT40- H7 cells by treatment with 300 ng/mL of doxycycline (Sigma-Aldrich, USA).
-
Cells were harvested and lysed in lysis buffer (25 mmol/L Tris–HCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% IGEPAL CA-630, 5% glycerol, 1 mmol/L PMSF, and a protease inhibitor cocktail). The lysates were centrifuged and supernatants were collected. Protein concentration in the supernatant was measured with a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific). Equal amounts of total protein were electrophoresed on a sodium dodecyl sulfate–polyacrylamide gel and transferred to a nitrocellulose membrane (Pall). After blocking with 5% nonfat milk, the membrane was sequentially incubated with primary antibodies and IRDye-conjugated secondary antibodies. Finally, the membranes were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences, USA) according to the manufacturer's instructions.
-
The frequency of gene conversion and point mutations in IgV was measured as previously described (Romanello et al. 2016). Briefly, genomic DNA was extracted and amplified using the Q5 Hot Start High-Fidelity DNA polymerase (New England Biolabs, USA). The rearranged Ig light chain V region (IgLV) was amplified with the primers LVF (5'-GGCTCTGTCCCATTGCTGCGCGG-3') and LVR (5'-CCCCAGCCTGCCGCCAAGTCCAAG-3'); the rearranged Ig heavy chain V region (IgHV) was amplified with the primers HVF (5'-GGCGGCTCCGTC AGCGCTCTCT-3') and HVR (5'-GCCGCAAATGATGG ACCGAC-3'). PCR products were cloned into the pEASY-Blunt Zero Vector (Transgene, China) and 50 transformed colonies were subjected to sequencing. Gene conversions and point mutations were analyzed as previously described (Sale et al. 2001).
-
Antigen-specific DT40 cells were selected using previously described methods (Seo et al. 2006), with some modifications. Briefly, the Zika virus NS1 protein (Sino Biological, China) was coupled to Dynabeads M280 Tosylactivated (Thermo Fisher Scientific). TSA-treated DT40-H7 cells were incubated with NS1-conjugated Dynabeads, and cells bound to the beads were selected using a MPC-S magnet (Thermo Fisher Scientific). The selected cells were cultured for 4 days and selection repeated once again. Then, single cell clones were isolated using the limited dilution method. After a week of incubation, the culture supernatants were analyzed using an enzyme-linked immunosorbent assay (ELISA) performed on microplates (Corning, USA) coated with antigens. A commercial rabbit polyclonal antibody against NS1 (GeneTex, USA) was used as a positive control. The concentration of chicken IgM in the culture supernatants was measured using a Chicken IgM ELISA kit (Abcam, UK) according to the manual.
-
Zika virus (ZIKV, strain GZ01/2016, GenBank: KU820898) was propagated and titrated in Vero cells, as previously described (Contreras and Arumugaswami 2016). For infection, Vero cells were incubated with Zika virus at an MOI of 0.1 in serum-free medium. Infected cells were collected to analyze the levels of NS1 protein at 3 days post-infection.
Cell Culture and Transfection
Generation of AID-inducible DT40 Cells
SDS-PAGE and Western Blotting
Analysis of Gene Conversion and Point Mutation in IgV
Selection of Antigen-specific DT40 Cells
Zika Virus and Infection
-
Since the expression of AID gene should be tightly regulated when using DT40 cells for screening monoclonal antibodies, we generated an AID-inducible DT40 cell line. First, the AID gene was knocked out in DT40 cells using the CRISPR/Cas9 system, as described in the Materials and methods section. An AID-deficient DT40 cell line, named DT40-dAID, was isolated and the knockout of AID was confirmed using Western blotting (Fig. 1A). Genome sequencing was performed to further validate AID deficiency. One allele of the AID gene in DT40-dAID cells contained a 7 base-pair deletion and the other allele contained a 94 base-pair deletion. Both deletions resulted in frame-shift mutations (Fig. 1B).
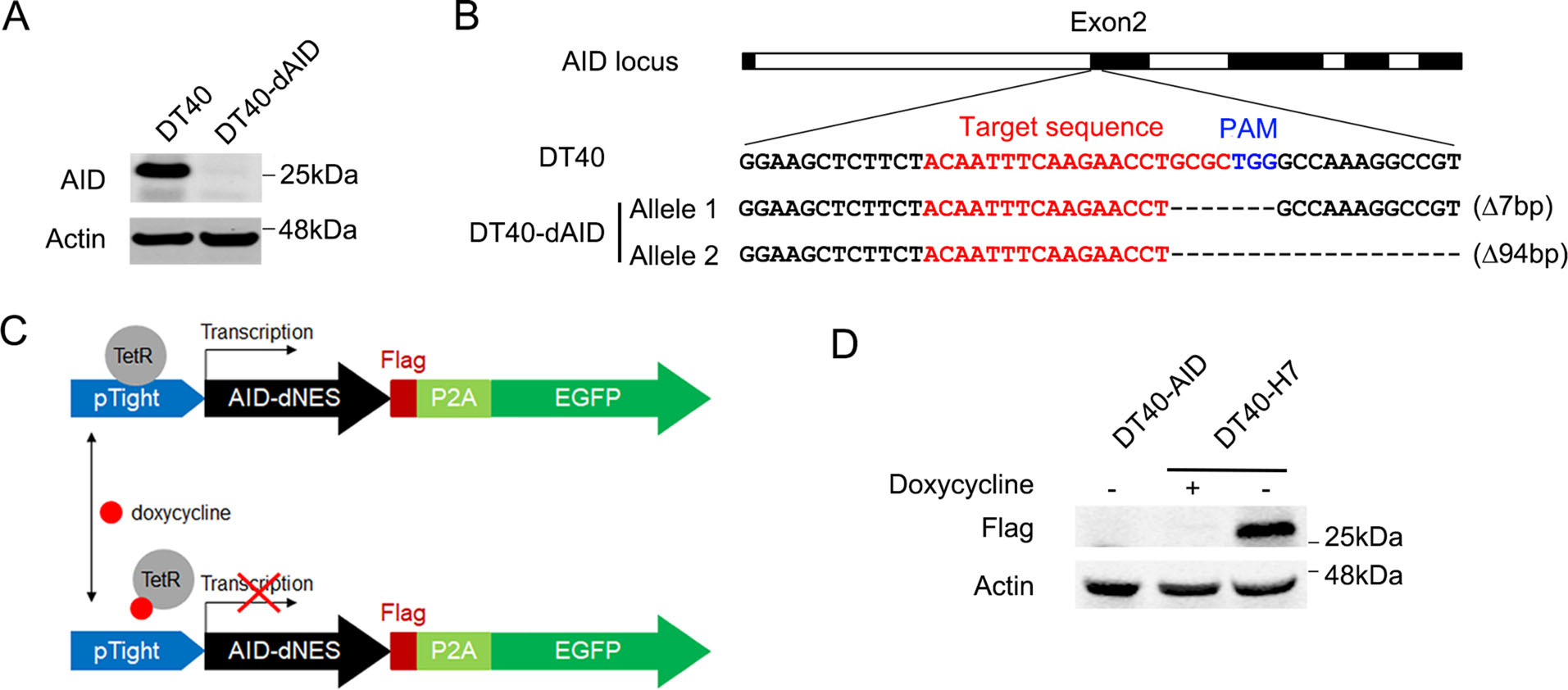
Figure 1. Generation of an AID-inducible DT40 cell line, DT40-H7. A The endogenous AID gene was knocked out in DT40 cells using the CRISPR/Cas9 genome editing system. Disruption of AID was confirmed in the AID-deficient cell line, DT40-dAID, using Western blotting. B Sequences of the edited AID gene in the DT40-dAID cell line are shown. C The strategy used to induce AID expression in DT40-dAID cells: the nuclear export signal (NES) at the C-terminus of AID was replaced with a Flag tag. An EGFP sequence linked by a 2A peptide was fused to the Flag tag. Expression of AID-dNES/Flag/ EGFP was driven by the pTight promoter. In the absence of doxycycline, TetR bound to the pTight promoter and activated the transcription of AID-dNES. In the presence of doxycycline, TetR was released from the promoter and AID-dNES expression was inhibited. D A stable cell line, expressing TetR and harboring the pTight/AID/ P2A/EGFP expression cassette, was isolated (DT40-H7). The expression of AID-dNES was analyzed using Western blotting.
Thereafter, the Tet-Off system was introduced to allow DT40-dAID cells to express an exogenous AID protein (Fig. 1C). We expressed a truncated AID protein, in which the nuclear export signal located at the C-terminus was deleted (AID-dNES) to increase the AID-induced hypermutation frequency (Magari et al. 2010). Since the antibody against AID recognized the C-terminus of AID, the exogenously expressed AID-dNES was not detected by the AID antibody. A Flag tag was fused to AID-dNES to measure AID-dNES levels using Western blotting. Enhanced green fluorescent protein (EGFP) linked by a 2A peptide (Kim et al. 2011) was expressed downstream of AID-dNES to analyze the expression of AID-dNES using flow cytometry. The expression of AID-dNES/P2A/EGFP was driven by the pTight promoter and regulated by the Tet-responsive transactivator (TetR) (Gossen and Bujard 1992). A stable cell line, expressing TetR and harboring the pTight/AID/P2A/EGFP expression cassette, was isolated (DT40-H7).
In the absence of doxycycline, TetR binds to the pTight promoter and activates the transcription of AID-dNES. In presence of doxycycline, TetR is released from the promoter and AID-dNES expression is inhibited (Fig. 1C). As shown in Fig. 1D, AID-dNES levels in untreated DT40-H7 cells were detected using Western blotting. After the treatment with doxycycline, AID-dNES expression was abolished.
-
We proceeded to analyze whether the induction of AIDdNES expression in DT40-H7 was reversible (Fig. 2). In the absence of doxycycline, 99.5 ± 0.3% of the DT40-H7 cells were GFP-positive, as determined using flow cytometry. After treatment with doxycycline for 2 days, > 99% of the cells were GFP-negative. Further sorting or selection was not required to obtain the high percentage of cells that switched from GFP-positive to GFP-negative. However, the Cre-loxP system has been reported to require puromycin selection to achieve a similar percentage of cells displaying this change (Kanayama et al. 2005). Subsequently, doxycycline was removed from the culture and the cells were continuously cultured for 5 more days. The percentage of GFP-positive cells was 96.5 ± 2.1%, which was much more efficient than the Cre-loxP system and guaranteed the resumption of hypermutations in the selected primary clones.
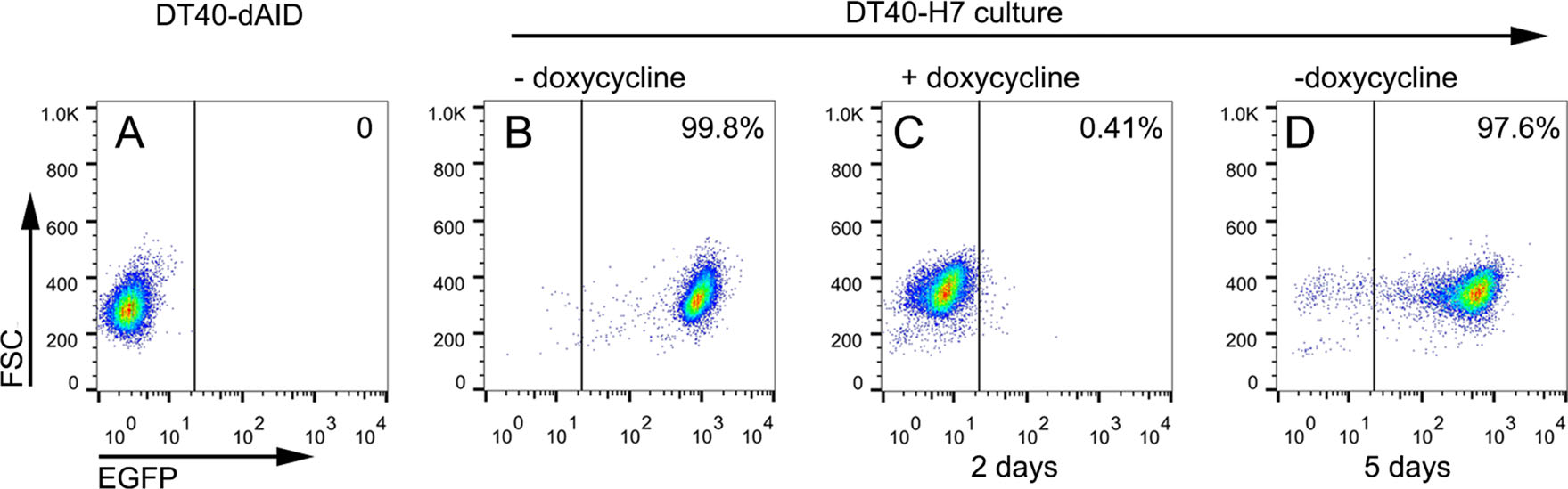
Figure 2. Reversible induction of AID expression in DT40-H7 cells. A DT40-dAID cells were used as a negative control. B The culture was started with DT40-H7 cells that were not treated with doxycycline. AID expression was analyzed by measuring EGFP levels by flow cytometry. C Cells were then treated with 0.625 ng/mL of doxycycline for 2 days and EGFP expression was analyzed. Subsequently, doxycycline was removed from the culture. D The cells were continuously cultured for another 5 days and EGFP expression was analyzed.
-
Since gene conversion and point mutations in DT40 cells require the expression of AID, we investigated whether the hypermutations were controlled by the induction of AIDdNES expression in DT40-H7 cells. DT40-H7 cells were cultured for 2 months in presence or absence of doxycycline, and IgLV gene was subsequently sequenced as described above. No mutation was identified in DT40- dAID cells and doxycycline-treated DT40-H7 cells, whereas mutations were detected in 26 of the 50 clones of DT40-H7 cells that were sequenced (Fig. 3A). Each of the 26 clones contained a unique IgLV sequence, suggesting that the cultured DT40-H7 cells might display a high Ig diversity. The distribution of gene conversion and point mutations in the IgLV gene in a portion of the sequenced clones is shown in Fig. 3B.
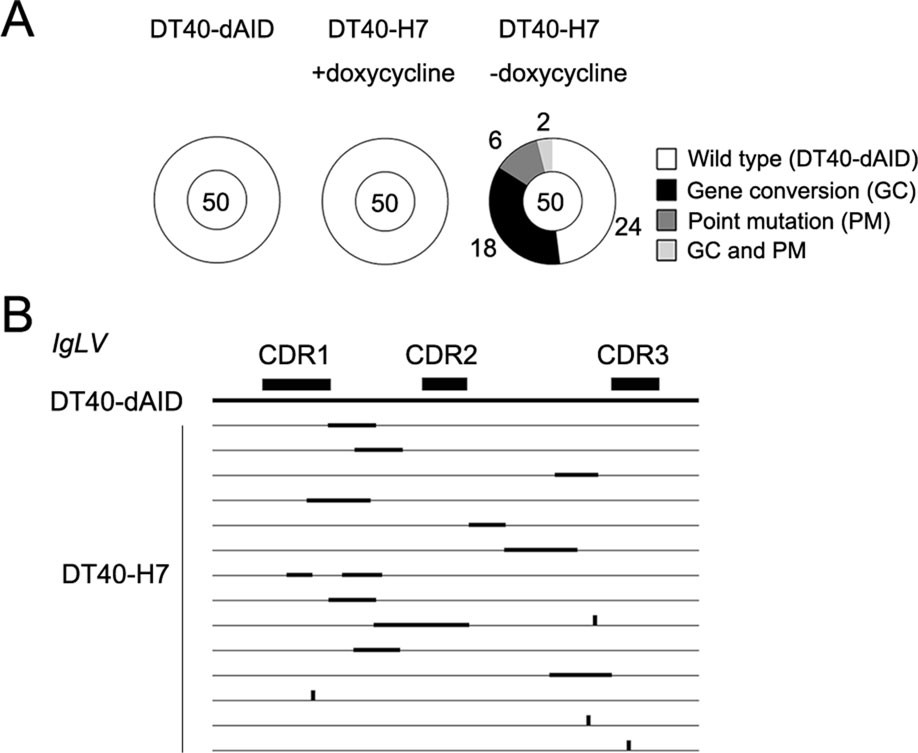
Figure 3. Hypermutations in cultured DT40-H7 cells. DT40-dAID cells, doxycycline-treated DT40-H7 cells, and untreated DT40-H7 cells were cultured for 2 months before sequencing IgLV. A Proportion of clones carrying different types of mutations in IgLV. The total number of sequenced clones is shown in the center of the pie chart. The segment sizes are proportional to the number of clones (indicated around the pie chart) carrying different types of mutations. B The distribution of gene conversions and point mutations in the IgLV gene in a portion of the sequenced clones is shown. Thick horizontal lines indicate gene conversions. Vertical lines indicate point mutations.
-
Antibody selection using the ZIKV NS1 protein was performed to clarify whether the DT40-H7 cells would be useful for antibody preparation. After a two-month culture, the expression of AID-dNES in DT40-H7 cells was inhibited by doxycycline. Using the methods described above, 25 of the 60 isolated cell clones showed positive results in ELISA (data not shown). IgLV and IgHV in the 25 positive clones were sequenced, and 3 unique clones (NS1-1, -17, and -18) were identified. Culture supernatants of the three clones were diluted to contain 1 μg/mL of sIgM and then applied to ELISA (Fig. 4A). Clone NS1-17 showed the highest absorbance value. Subsequently, the NS1-17 antibody was tested using Western blotting. As shown in Fig. 4B, the NS1-17 antibody recognized both the recombinant NS1 protein and NS1 protein in ZIKVinfected Vero cells.
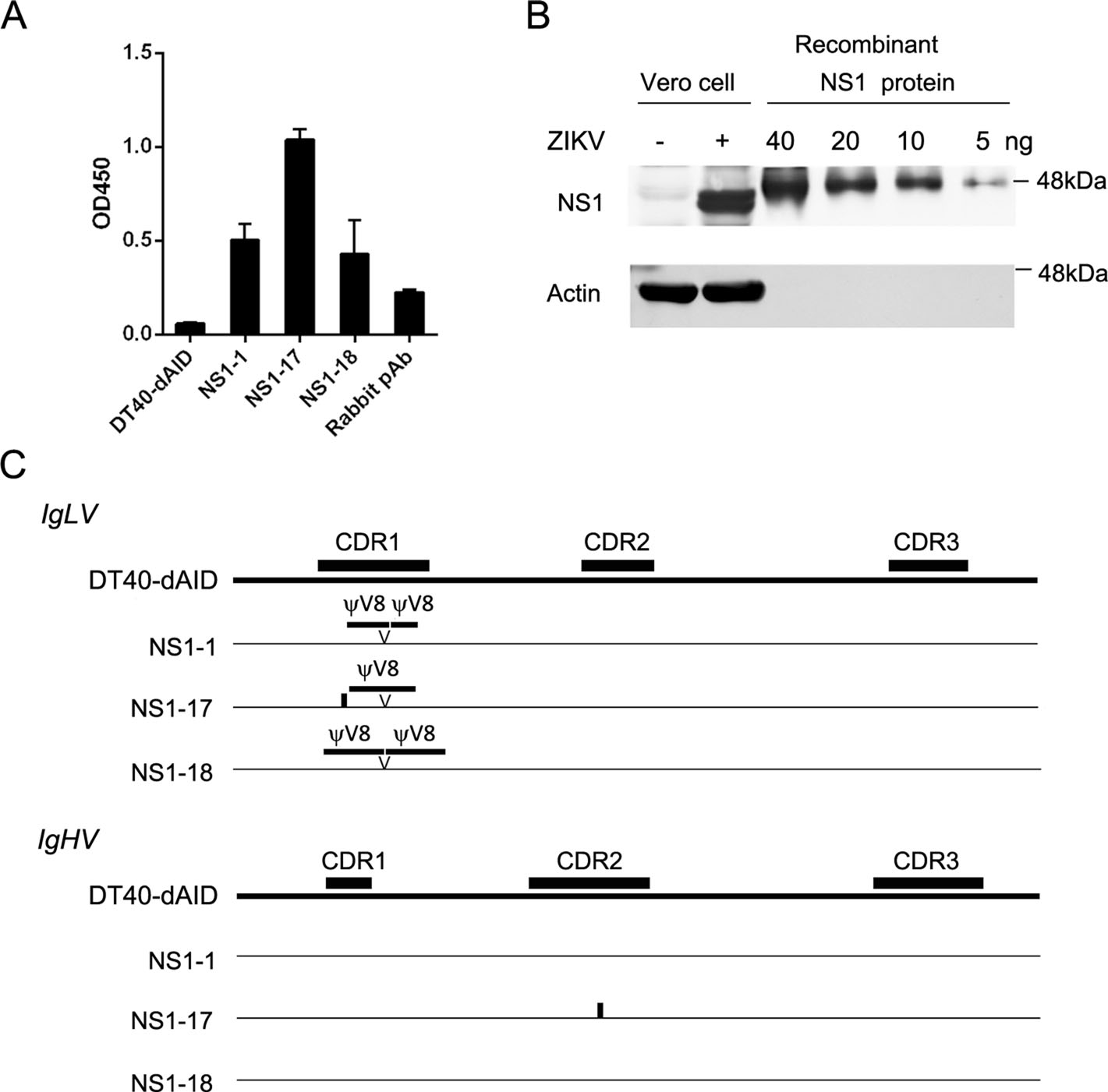
Figure 4. Selection of antibodies against the ZIKV NS1 protein. After the selection of cultured DT40-H7 cells, three unique clones expressing IgM against the ZIKV NS1 protein were obtained. A The concentration of sIgM in the supernatants from the three clones (NS1-1, - 17, and -18) was adjusted to 1 μg/mL and the diluted supernatant was applied to NS1 ELISA. A commercial rabbit polyclonal antibody (0.1 μg/ mL) against NS1 was used as a positive control. B Western blot showing levels of the total protein from ZIKV-infected Vero cells and the recombinant NS1 protein detected using the NS1-17 supernatant. C Comparison of the IgLV and IgHV sequences from NS1-1, - 17, and -18 clones. Triangles and thick horizontal lines indicate duplications of V pseudogene donors. Vertical lines indicate point mutations.
The IgLV and IgHV sequences of the three clones were then analyzed (Fig. 4C), and ψ8 V duplications were identified at the same position in the CDR1 region of IgLV gene in all three clones compared to that in the parental DT40-dAID cells. In addition to a duplication in IgLV, the NS1-17 clone contained point mutations in IgLV (a Serine to Threonine mutation at the 9th amino acid residue of CDR1) and IgHV (a Glycine to Serine mutation at the 8th amino acid residue of CDR2).
Generation of an AID-inducible DT40 Cell Line
Reversible Induction of AID Expression in DT40-H7 Cells through Doxycycline Treatment
Hypermutations in Cultured DT40-H7 Cells
Selection of Antibodies against the ZIKV NS1 Protein
-
The DT40 cell line produces IgM-type antibodies and undergoes hypermutations in the variable region of the immunoglobulin gene during culture, owing to which they form a natural antibody library. Furthermore, the fast growth of DT40 cells enables rapid antibody acquisition compared to that in hybridoma and phage display systems (Seo et al. 2006). Therefore, the DT40 antibody library is suitable for the rapid generation of diagnostic antibodies in emerging viral infections. In this study, we generated a new DT40 antibody library and obtained antibodies against ZIKV NS1 protein using this library.
Since hypermutation in DT40 cells is regulated by the AID gene, AID expression must be controlled to fix the Ig sequence by stopping mutation or to improve affinity by resuming mutation after selection of the antibodies. In a previous study, a Cre-loxP system-based switching device was knocked into the endogenous AID gene of DT40 cells (Kanayama et al. 2005). However, repeated switching of AID expression, using this approach, required sorting or selection with puromycin in addition to treatment with 4-hydroxytamoxifen. In our current study, control of AID expression in DT40-H7 cells was much simpler and efficient (Fig. 2).
After a two-month culture, DT40-H7 cells displayed a similar diversity as in previous studies (Magari et al. 2010). Using the antibody library, we obtained three monoclonal antibodies against ZIKV NS1 protein in only 2 weeks. Since the CDRs form the variable domain of antigen receptor of antibodies, their sequences determine the specificity of antibodies. Since ψ8 V duplications were identified at the same position in the CDR1 region of IgLV gene in all the three clones, it was suggested to contribute to NS1 specificity and that the three antibodies recognized the same epitope of NS1. Furthermore, this epitope might be a dominant one and cell clones recognizing this epitope might be enriched after the two rounds of antibody screening, thereby explaining why the three selected clones were quite similar. In addition to a ψ8 V duplication in IgLV, point mutations in IgLV and IgHV were identified in the NS1-17 clone, which displayed the highest affinity among the three antibodies (Fig. 4), suggesting that the point mutations might improve the affinity, as previously reported (Kajita et al. 2010).
In conclusion, Ig gene conversion and point mutations were tightly and efficiently controlled in DT40-H7 cells. This AID-inducible DT40 cell line is a useful tool for the selection and evolution of antibodies and may also be a powerful tool for the rapid selection and generation of diagnostic antibodies for emerging infectious diseases.
-
This work was sponsored by the Project of the National Defense Science and Technology Innovation Special Zone (to HH, 17-163-12-ZT-005-013-01), the CAMS Innovation Fund for Medical Sciences (to HH, CIFMS 2016-12 M-1-013 and to ZZ, CIFMS 2016-12 M-1-014 and 2016-12 M-3-020), the National Key Research and Development Program (to ZZ, 2016YFD0500300), and the Fundamental Research Funds for the Central Universities (to HH, 2018PT31032).
-
HH and ZZ designed the experiments. BW, FW, and HH carried out the experiments. BW and FW analyzed the data. HH wrote the paper. ZZ checked and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: