HTML
-
Human adenovirus (HAdV), which belongs to the genus Mastadenovirus, was first isolated from adenoids in 1953 (Rowe et al. 1953). So far, 103 types of HAdV (http://hadvwg.gmu.edu/), including candidates from HAdV-53 to HAdV-103, have been identified and classified into seven species (A to G) on the basis of phylogenomics, serology, and whole-genome sequencing (Jones et al. 2007; Seto et al. 2011). HAdV infections are one of the leading causes of severe respiratory tract diseases in pediatric populations and contribute significantly to the burden of disease in the elderly, immunocompromised individuals and organ transplant recipients (Ison 2006; Echavarria 2008; Khanal et al. 2018). HAdV-3 which has been identified in epidemics globally and causes acute respiratory disease, is associated with symptoms ranging from discomfort and/or influenza-like fever to pneumonia and/or death (Ryan et al. 2002; Lebeck et al. 2009).
The development of effective and safe neutralizing antibodies (NAbs) and vaccines against HAdV-3 remains an important goal. Additionally, a method for measuring the neutralization activity is needed in order to be able to evaluate an effective immune response against the virus. The traditional methods for assessing the neutralization activity of antibodies in samples are the microneutralization (MN) assay and the plaque reduction neutralization test (PRNT). However, because of the insufficient sensitivity of the MN assay, it is not suitable for measuring neutralization in samples with a low NAb concentration. The PRNT is not suitable for the high-throughput screening of large collections of samples. To overcome the limitations of these two assays, some reporter genes have been used, such as green fluorescent protein (GFP) (Johnson et al. 2008; van Remmerden et al. 2012; Lin et al. 2017), b-galactosidase (Cheng et al. 2002), luciferase (Luc) (Fuentes et al. 2013) and secreted alkaline phosphatase (SEAP) (Bourne et al. 2005; Kaku et al. 2012). For example, when Luc and SEAP were used in conjunction with a chemiluminescent substrate, the sensitivity of the chemiluminescence-based assay was reportedly 10-fold greater than that of the fluorescence-based assay, and 80–100-fold greater than that of a colorimetric method (Yang et al. 1997). The fluorescence-based assay detected aggregate cell infection, as indicated by the expression of recombinant GFP. The use of recombinant GFP expression coupled with flow cytometry allowed the production of readouts from individual cells, which advanced the sensitivity of the assay (Earl et al. 2003; Cosma et al. 2004). The flow cytometry-based neutralization (FCN) assay using GFP-expressing recombinant viruses or pseudoviruses has become more common and better standardized as a new technology for measuring antibody neutralization (Pierson et al. 2006; Sashihara et al. 2009).
Herein, we describe the development of a sensitive and high-throughput flow cytometry-based assay for measuring the neutralization activities of sera, cell culture supernatants, and chimeric antibodies against HAdV-3 on the basis of an recombinant HAdV-3 (rHAdV-3) construct expressing the EGFP. We report a comparison of the newly established FCN assay with the MN assay using panels of human sera, where the results demonstrated a linear correlation between the two assays. In addition, the FCN assay was shown to be a sensitive and high-throughput method that worked efficiently for screening anti-HAdV-3 NAbs in cell culture supernatants.
-
A549 cells, Chinese hamster ovary (CHO) cells, and 3D7 cells (Liu et al. 2014) (a mouse hybridoma that expresses anti-HAdV-3 NAb) were separately cultured in Dulbecco's modified Eagle medium/nutrient mixture F-12 (DMEM/F- 12) (GIBCO BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated (56 ℃, 30 min) fetal bovine serum (GIBCO BRL, Grand Island, NY, USA) and antibiotics (100 U/mL penicillin, 100 lg/mL streptomycin) (GIBCO BRL, Grand Island, NY, USA) at 37 ℃ in 5% CO2. The recombinant HAdV-3 expressing the EGFP (rHAdV-3/EGFP) was constructed as previously described (Zhang et al. 2009), and cultured and titrated separately in A549 cells. The 50% tissue culture infectious dose (TCID50) of the virus was calculated using the ReedMuench method, based on cytopathic effects (CPEs) (Reed and Muench 1938). The TCID50 was converted to an multiplicity of infection (MOI) value using the following formula: MOI = [(plaque forming unit/mL (TCID50/mL × 0.69)) × (volume of inoculum added to cells)]/(total number of cells in well) (Nyberg-Hoffman et al. 1997).
-
In total, 72 heat-inactivated (56 ℃, 30 min) adult human serum samples collected from blood donors were obtained from Guangzhou Blood Centre (Guangzhou, China).
-
Total RNA was extracted from approximately 5 × 106 3D7 cells using the EasyPure® RNA Kit (TransGen, Beijing, China), and cDNA was synthesized from the total RNA using the TransScript® II All-in-One First-Strand cDNA Synthesis SuperMix for PCR (TransGen, Beijing, China). PCR cloning of the immunoglobulin V regions was performed using PrimeSTAR® HS DNA Polymerase with GC Buffer (TaKaRa, Tokyo, Japan) as previously described (Calvert et al. 2016). The chimeric antibody from the serum-free culture supernatant of CHO cells, which were cotransfected with paired immunoglobulin heavy- and light-chain gene expression vectors (pfusess-hchg1 and pfuse2ss-hclk, InvivoGen, San Diego, CA, USA) containing the variable regions in a 6-well plate according to the Lipofectamine® LTX & PLUSTM reagent protocol 2013 (Invitrogen, Carlsbad, CA, USA), was assessed for its neutralization activity. In brief, CHO cells were washed three times with DMEM/F-12 and then mantained in OptiMEM® reduced-serum medium (GIBCO BRL, Grand Island, NY, USA) before transfection. The CHO cells were incubated for 3 days at 37 ℃ in 5% CO2 after cotransfection, before the assay for transgene expression. Negative control (pfusess-hchg1 and pfuse2ss-hclk) was prepared.
The expression analysis and purification of the chimeric antibody were performed by Atagenix Laboratories Co., Ltd. (Wuhan, China). In brief, plasmids were transfected with 80 mL of 293F cells. The culture medium was collected when the cell viability dropped below 50% after transfection. The chimeric antibody in the culture medium was purified using Protein G Resin. After the filtered culture medium had been passed through, the column was washed thoroughly with phosphate-buffered saline (PBS, pH 7.5), and the bound chimeric antibody was eluted with an elution buffer (100 mmol/L glycine, pH 2.7). The purified chimeric antibody solution was further concentrated, and buffer exchange with PBS (pH 7.5) was then conducted by ultrafiltration. The chimeric antibody concentration was measured spectrophotometrically.
-
The purified chimeric antibody was preheated at 100 ℃ for 10 min and were separated and resolved by 10% SDSPAGE under denaturing and reducing conditions. Staining was performed using BeyoBlueTM Coomassie Blue Super Fast Staining Solution (Beyotime Biotechnology, Shanghai, China) for 1 h, and the gel was destained in distilled water overnight. SDS-PAGE was used to analyze the purity of the purified chimeric antibody.
-
The anti-HAdV-3 NAb titers in 72 serum samples were detected with the MN assay. For this, serial twofold dilutions of the serum (using DMEM/F-12 and beginning with a dilution of 1:9) in 96-well plates were mixed with an equal volume of the rHAdV-3/EGFP stock (containing 200 TCID50/100 μL) and the virus-serum mixtures were incubated for 1 h at 37 ℃ in 5% CO2. The serum titers ranged from 1:18 to 1:9216. Subsequently, the virus-serum mixture was inoculated in triplicate in a volume of 100 μL per well onto A549 cell monolayers (80% confluent cultures) contained in the wells of 96-well plates. After 2 h of incubation at 37 ℃ in 5% CO2, the culture supernatant in each well was removed. Then, 100 μL of DMEM/F-12 was added to each well and the cells were maintained at 37 ℃ in 5% CO2. After 3 days, the cells were observed to evaluate the appearance of CPEs. Each test included control wells of the same negative and positive sera, uninfected cells, and virus only. The highest dilution of the sera that completely prevented CPEs in 50% of the wells was defined as the neutralization titer.
-
To investigate the influence of the cell confluence on the percentage of EGFP-expressing cells, infection of a confluence titration of A549 cells was performed. For this, cell monolayers of 70%, 80%, 90%, and 100% confluence were infected separately with the virus (100 TCID50/100 μL) in a volume of 100 μL per well in 96-well plates. All measurements were in quadruplicates. After 2 h of incubation at 37 ℃ in 5% CO2, the culture supernatant was removed. Then, 100 μL of DMEM/F-12 was added to each well and the cells were maintained at 37 ℃ in 5% CO2. At 24 h post-infection, the cells were harvested and fixed for flow cytometric analysis (NovoCyte, ACEA Biosciences, San Diego, CA, USA), because this time point is too early for the cell-to-cell spread of newly assembled virus particles (Berk 2007); shorter duration infections resulted in fewer EGFP-positive cells. Infection was monitored as a function of EGFP expression. Prior to the flow cytometric assessment, the cells were treated with 0.25% trypsin–EDTA (GIBCO BRL, Grand Island, NY, USA) to ensure a singlecell suspension for optimal analysis and then fixed with 4% paraformaldehyde. The flow cytometry-based confluence titration assay was performed in 96-well plates with flatbottomed wells.
To convert the TCID50 to an MOI value, the rHAdV-3/ EGFP stock (104.5 TCID50/100 μL) was quantified by flow cytometric titration of the infected A549 cells (NovoCyte, ACEA Biosciences, San Diego, CA, USA). For this, twofold serial dilutions of the virus (using DMEM/F-12 and beginning at 1:10 dilution) were inoculated in quadruplicate in a volume of 100 μL per well onto A549 cell monolayers (~3.4 × 104 cells/well at 90% confluence) in 96-well plates. After 2 h of incubation at 37 ℃ in 5% CO2, the culture supernatant was removed. Then, 100 μL of DMEM/F-12 was added to each well and the cells were maintained at 37 ℃ in 5% CO2. Infection was monitored as a function of EGFP expression at 24 h post-infection, using flow cytometry. The cells were treated in the same way as for the protocol of the flow cytometry-based confluence titration assay.
The antibody neutralization of rHAdV-3/EGFP was measured using the FCN assay, where a range of MOI was used. (1) For the assessment of the neutralization activity of the purified chimeric antibody, serial dilutions of the antibody (using DMEM/F-12 and beginning with a dilution of 1:2000) were mixed with an equal volume of the rHAdV-3/EGFP stock (containing 200 TCID50/100 μL). Furthermore, to ascertain whether the newly established FCN assay follows the percentage law, four dilutions of rHAdV-3/EGFP at MOIs of 3.2 × 10-2, 8 × 10-3, 4 × 10-3, and 5 × 10-4 were separately evaluated with serial dilutions of the chimeric antibody (using DMEM/F- 12 and beginning with a dilution of 1:2000). Control wells of uninfected cells and virus only were included. The effective dilution ratio was determined as the highest dilution of the chimeric antibody that resulted in 50% or more reduction of the average percentage of EGFP-expressing cells relative to that with the control well of virus only. (2) To detect the anti-HAdV-3 NAb titers in the 72 serum samples, the same serum dilution ratios as used for the MN assay were applied. The same infectious dose (100 TCID50/100 μL) of rHAdV-3/EGFP at an MOI of 2 × 10-3 as used for the MN assay was used. Each test included control wells of the same negative and positive sera, uninfected cells, and virus only. The endpoint neutralization titer was defined as the highest dilution of the serum that resulted in 50% or more reduction of the average percentage of EGFP-expressing cells relative to that with the control well of virus only. (3) For the assessment of the neutralization activity of the chimeric antibody from the serum-free culture supernatant of CHO cells, non-diluted and 1:2-diluted cell culture supernatants were used. A dilution of rHAdV-3/EGFP at an MOI of 6.4 × 10-4 was used. Control wells of negative control, uninfected cells, and virus only were included. The serial dilutions of the purified chimeric antibody, serial dilutions of the serum, and non-diluted and 1:2-diluted cell culture supernatants (using DMEM/F-12) were separately mixed with an equal volume of the rHAdV-3/EGFP stock. After 1 h of incubation at 37 ℃ in 5% CO2, the virus-serum mixture, virus-cell culture supernatant mixture, the viruschimeric antibody mixture were separately inoculated (in triplicate in a volume of 100 μL per well) onto A549 cell monolayers (of 90% confluence) in 96-well plates. After 2 h of incubation at 37 ℃ in 5% CO2, the mixture was removed. Then, 100 μL of DMEM/F-12 was added to each well and the cells were maintained at 37 ℃ in 5% CO2. Infection was monitored as a function of EGFP expression, using flow cytometry. After 24 h, the cells were treated in the same way as for the protocol of the flow cytometrybased titration assay for rHAdV-3/EGFP.
-
Correlations between the anti-HAdV-3 NAb titers of the 72 serum samples detected separately by the MN assay and the FCN assay were evaluated by Spearman's rank test using GraphPad Prism (Version 7.04; GraphPad Software Inc., La Jolla, CA, USA), and a P value of less than 0.05 was considered to be statistically significant.
Cells and Viruses
Human Serum Samples
Chimeric Antibody
SDS-PAGE
MN Assay
FCN Assay
Statistical Analysis
-
With increasing confluences of A549 cell monolayers, decreasing percentages of EGFP-expressing cells were detected by flow cytometry. When cell monolayers were at 90% and 100% confluences, there was little different between them in the percentage of EGFP-expressing cells (Fig. 1A). Cell monolayers of 90% confluence per 96-well were selected for all subsequent flow cytometry-based assays. The MOI of rHAdV-3/EGFP was 0.64. When A549 cells were infected with low to high dilutions of rHAdV-3/ EGFP at different MOIs, decreasing percentages of EGFPexpressing cells were detected by flow cytometry. A curve describing the relationship between the MOIs and the percentages of EGFP-expressing cells was drawn (Fig. 1B).
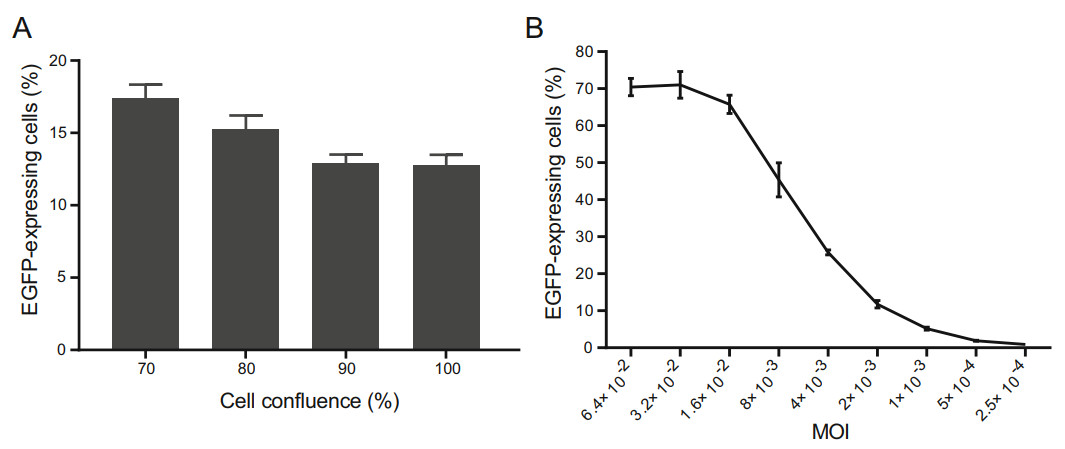
Figure 1. Optimization of the cell confluence and titration of rHAdV-3/ EGFP using flow cytometry. A The percentage of EGFP-expressing cells is affected by the cell confluence. Cell monolayers at different levels of confluence were infected separately with the same dose of rHAdV-3/EGFP. B Curve showing the relationship between the virus input and the percentage of EGFP-expressing cells. A549 cells were infected with twofold serial dilutions of rHAdV-3/EGFP (beginning at 1:10 dilution) at different MOIs. The percentage of EGFPexpressing cells was determined by counting the number of infected cells and dividing this number by the total number of cells. The results represented the mean ± SD of four measurements.
-
The genes encoding the variable regions of immunoglobulin heavy- and light-chains from 3D7 cells were amplified and cloned into expression vectors. The chimeric antibody, which belongs to IgG1, j, was expressed in 293F cells. The concentration of the purified chimeric antibody was 2.3 mg/mL. The purified chimeric antibody was verified by SDS-PAGE, which showed two bands corresponding to the predicted molecular weights of the light chain and the heavy chain, respectively (Supplementary Figure. S1). The SDS-PAGE results showed that the purity of the chimeric antibody was over 95%.
-
The rHAdV-3/EGFP was incubated with serial dilutions of the purified chimeric antibody (the dilution ratios refer to Fig. 2) prior to the infection of A549 cells. After addition of the chimeric antibody, the dilution of the virus at an MOI of 2 × 10-3 was applied. Incubation with the chimeric antibody with the 1:12, 000 dilution blocked more than 50% of the virus infection. Thus, the result demonstrated that the chimeric antibody was an anti-HAdV-3 NAb.
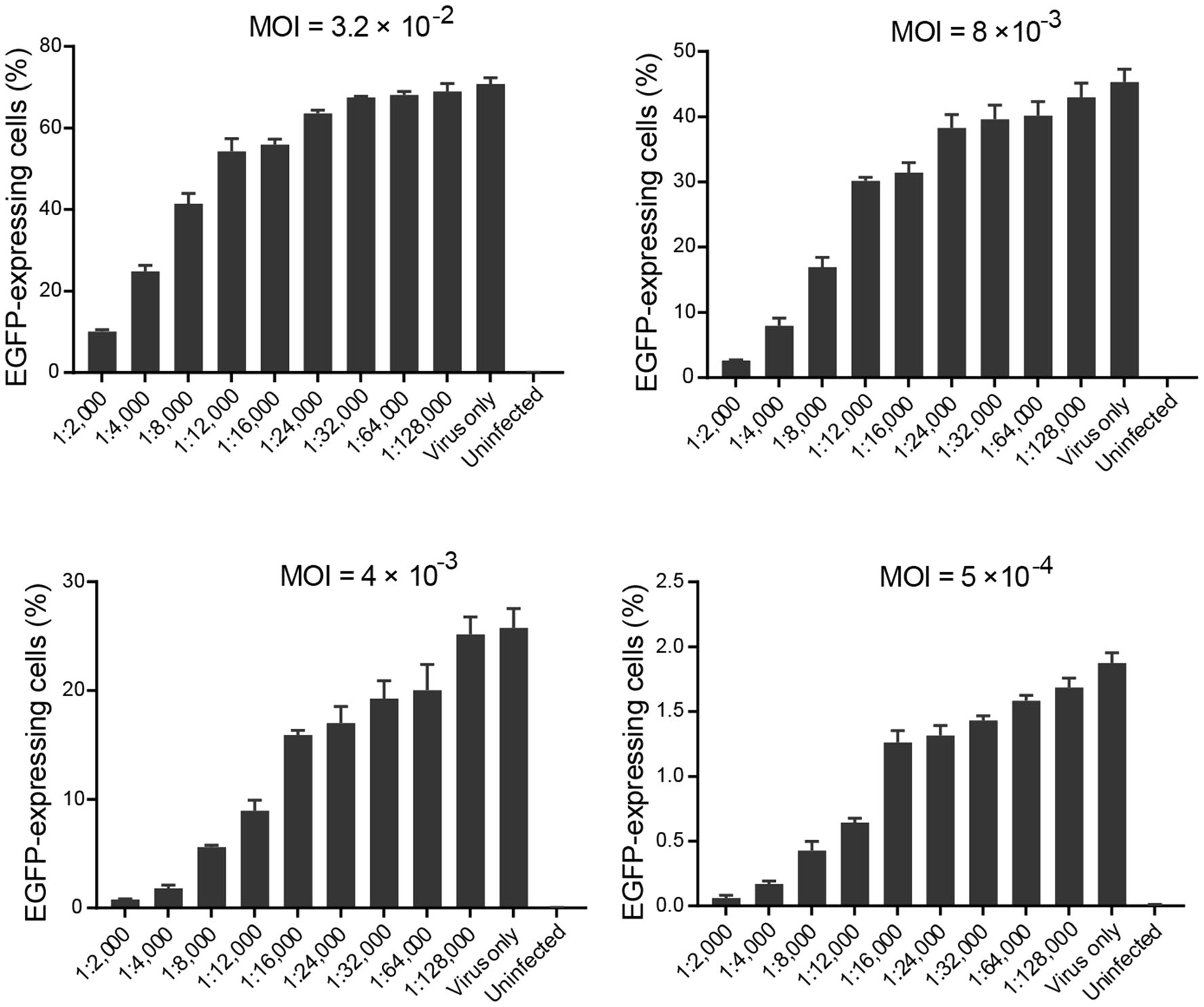
Figure 2. Bar graphs depicting the relationship between the purified chimeric antibody dilution ratios and the percentage of EGFPexpressing cells over a wide range of MOI. The percentage of EGFPexpressing cells was determined by counting the number of infected cells and dividing this number by the total number of cells. Four dilutions of rHAdV-3/EGFP at MOIs of 3.2 × 10-2, 8 × 10-3, 4 × 10-3, and 5 × 10-4 (i.e., that would result in an average of 70.74%, 45.32%, 25.78%, and 1.88% infection of cells, respectively) were evaluated with serial dilutions (ranging from 1:2, 000 to 1:128, 000) of the purified chimeric antibody. Each chimeric antibody dilution was tested in triplicate; the results represented the mean ± SD of three measurements. The presence of the chimeric antibody had resulted in a reduction of the average percentage of EGFPexpressing cells relative to that with the control well of virus only. The average percentage of EGFP-expressing cells increased when the chimeric antibody was diluted from 1:2000 to 1:128, 000.
The percentage law states that the titer of NAb is not affected by the amount of virus present if the NAb is in excess of the virus (Andrewes and Elford 1933; Brioen and Boeye 1985). Assay compliance with the percentage law provides further reassurance that the assay result is not related to quantity of virus present. To ascertain whether the newly established FCN assay follows the percentage law, four dilutions of rHAdV-3/EGFP at MOIs range from 3.2 × 10-2 to 5 × 10-4 were evaluated with serial dilutions of the chimeric antibody with testing in triplicate and data is further described in detail (Fig. 2). The effective dilution ratios of the chimeric antibody that inhibited infectivity by 50%, evaluated with four dilutions of the virus at MOIs of 3.2 × 10-2, 8 × 10-3, 4 × 10-3, and 5 × 10-4, were 1:4000, 1:8000, 1:12, 000 and 1:12, 000, respectively (Fig. 2). In the assessment of variables, the neutralization titer of the chimeric antibody was not altered by MOIs range from 4 × 10-3 to 5 × 10-4. The experiment was repeated three times with similar results. A representative result is shown in the Fig. 2. While the assay follows the percentage law, it is noted that when the dilution of the virus at an MOI of 2.5 × 10-4 (i.e., that would result in an average of 0.93% infection of cells) was used, the percentage of EGFP-expressing cells of three wells at low antibody dilution displayed high variability that the measurement becomes unreliable. Therefore, it was not suitable as the viral infection rate in the control well for neutralization determining. To achieve assay precision and accuracy and ensure credible and biologically meaningful results, an MOI of not less than 5 × 10-4 was used for all subsequent flow cytometry-based assays.
-
A comparison of the MN and FCN assays was carried out by using them to detect the anti-HAdV-3 NAb titers in 72 serum samples. Eighteen independent pairs of results were obtained from the tested serum samples (Fig. 3). An NAb titer of less than 18 was matched to the position of 0 on the graph (Fig. 3). A linear correlation (R2 = 0.8911) with high statistical significance (P < 0.0001) was observed between the NAb titers detected separately by the MN and FCN assays, indicating that the two assays had similar effectiveness in measuring the NAb titers in serum samples (Fig. 3). On the basis of the established formula, the mutual conversion relationship of NAb titers detected separately by the MN assay and the FCN assay was developed (Fig. 3). The FCN assay required only 1 day to determine the titers by analyzing the average percentage of EGFPexpressing cells, whereas the MN assay required 3 days based on the observation of CPEs.
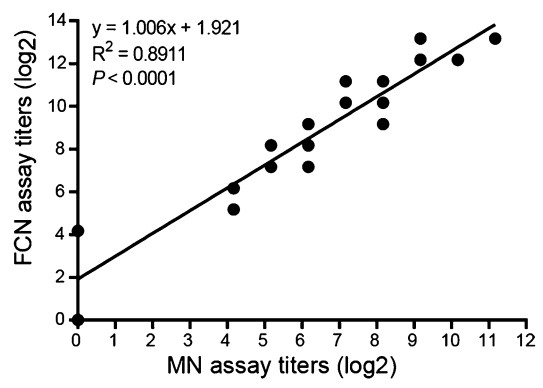
Figure 3. The strong correlation between the anti-HAdV-3 NAb titers measured separately by the FCN assay and the MN assay, using a panel of human sera. A total of 72 human serum samples were tested separately by the two assays, and 18 independent pairs of results were obtained. Each point represents one independent pair of results. The linear regression analysis demonstrated significant correlation; R2 = 0.8911, P < 0.0001.
-
The chimeric antibody from the serum-free culture supernatant of CHO cells was assessed for its neutralization activity. The rHAdV-3/EGFP was incubated in the presence of undiluted or 1:2-diluted cell culture supernatants prior to infection of the A549 cells. Analysis of the percentage of EGFP-expressing cells indicated that the presence of NAbs in both concentrations of cell culture supernatants had resulted in a reduction in the number of A549 cells infected by rHAdV-3/EGFP compared with that in the negative control (Fig. 4). The average percentage of EGFP-expressing cells was 1.02% higher with the 1:2-diluted cell culture supernatant than with the undiluted one. Incubation of the undiluted cell culture supernatant with the rHAdV-3/EGFP dilution that cause a 2.96% infection rate blocked more than 80% of virus infection, whereas the negative control had no inhibitory effect to the infection control (Fig. 4). The lower average percentage of EGFP-expressing cells in tests with the 1:2-diluted cell culture supernatant than that in the negative control indicated that the supernatant had prevented a small amount of virus from infecting the cells, although it blocked less than 50% of virus infection (Fig. 4).
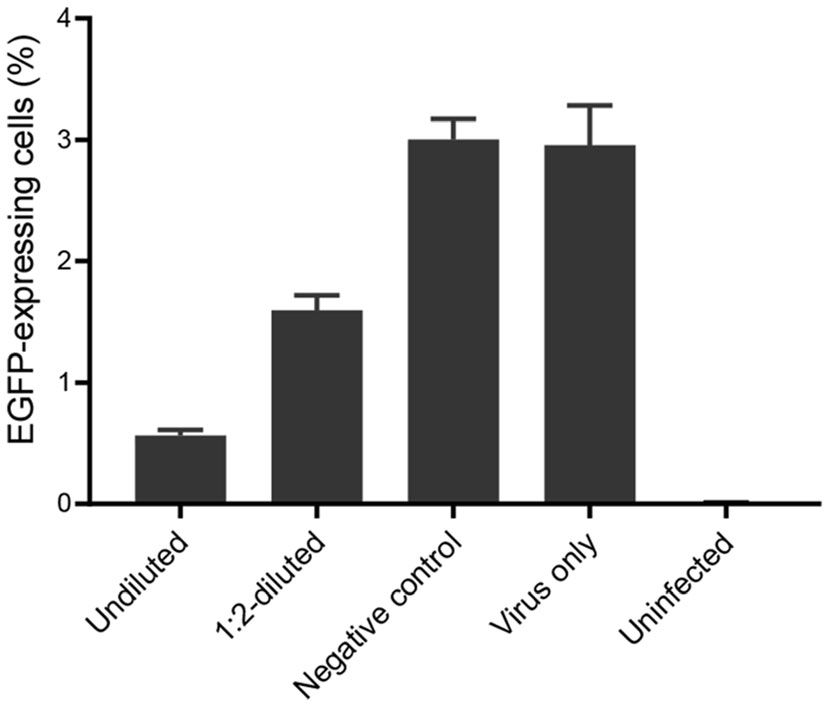
Figure 4. Neutralization test of rHAdV-3/EGFP by cell culture supernatants using FCN assay. The percentage of EGFP-expressing cells was determined by counting the number of infected cells and dividing this number by the total number of cells. Undiluted and 1:2-diluted serum-free culture supernatants of CHO cells were tested separately, in triplicate; the results represented the mean ± SD of three measurements.
Titration of rHAdV-3/EGFP Using Flow Cytometry
Chimeric Antibody Expression and Purification
Measurement of Neutralization by the Purified Chimeric Antibody Using the FCN Assay
Comparison of the MN Assay with the FCN Assay
Measurement of Neutralization by Cell Culture Supernatants Using the FCN Assay
-
EGFP-, Luc-, or SEAP-expressing HAdVs were previously constructed to improve the read-out of the MN assay (Sprangers et al. 2003; Aste-Amezaga et al. 2004; Zheng et al. 2017). The chemiluminescence-based neutralizing antibody detection test using rHAdV-5/Luc for determining anti-HAdV-5 NAb titers was reported to have higher sensitivity than the fluorescence-based neutralizing antibody detection test using rHAdV-5/EGFP (Liu et al. 2012). It had been reported that recombinant GFP expression coupled with flow cytometry for detecting NAbs improved the test sensitivity over that of the fluorescence-based neutralizing antibody detection test (Earl et al. 2003; Cosma et al. 2004). In this study, we developed a sensitive and high-throughput FCN assay for measuring the neutralization activities of sera, cell culture supernatants, and a chimeric antibody against HAdV-3 on the basis of an rHAdV-3 construct expressing EGFP. This newly established FCN assay has some advantages that circumvent the problems associated with the MN assay, such as the subjectively read-outs of the MN assay and its high laborintensiveness. In addition, the MN assay is time-consuming, as a single assay takes 5 days to complete. In contrast, the newly established FCN assay requires only 3 days for results to be obtained, and data analysis can be performed objectively and robotically. We have shown that the established FCN assay compares favorably with the MN assay. Thus, the FCN assay is a useful alternative to the MN assay for detecting anti-HAdV-3 NAb titers in serum samples.
A sensitive and high-throughput neutralization assay that is amenable to the validation and processing of a large number of samples would be of great value to NAb development. The high-throughput assessment of the neutralization activity of antibodies expressed in cell culture supernatants is essential for screening NAbs. However, because the concentration of antibodies in cell culture supernatants is low, the less-sensitive methods like the MN assay are not suitable for this purpose. To test the sensitivity of the FCN assay, the chimeric NAb expressed in the cell culture supernatant was used. Although a lower concentration of HAdV-3 in the inoculum was used in the MN assay for assessing the neutralization activity of the chimeric antibody expressed in the cell culture supernatant, an observable difference between the cell culture supernatant and the negative control, based on measurable CPEs on cell monolayers, could not be established (data not shown). By comparison, with the newly established FCN assay, the neutralization activity of the chimeric antibody expressed in the cell culture supernatant was determined on the basis of its reduction of the average percentage of EGFP-expressing cells relative to that in the negative control. Thus, this new FCN assay is sensitive enough and suitable for assessing the neutralization activity of antibodies in cell culture supernatants. In addition, our FCN assay is suitable for screening large panels of NAbs in cell culture supernatants using the 96-well plate format described herein, making it applicable for high-throughput screening. In short, with this newly established FCN assay, the screening of NAbs from large numbers of cell culture supernatants is possible.
On the basis of the neutralization activity measured with our FCN assay, it was determined that the chimeric antibody was an anti-HAdV-3 NAb. Subsequently, the chimeric antibody will be further transformed into different humanized antibodies. The use of cell culture supernatants expressing different humanized antibodies in the newly established FCN assay can be a rapid high-throughput screening for the neutralization of HAdV-3 without express and purify the NAbs separately. Furthermore, the neutralization activity of the different humanized anti-HAdV-3 NAbs can be compared accurately with the FCN assay instead of subjectively according to CPEs.
-
This study was supported by the National Science and Technology Major Project of China (2017ZX10103011-003, 2018ZX1010 2001), the National Natural Science Foundation of China (31570163) and Guangzhou Science and Technology Program key projects (201803040004).
-
ZL and RZ conceived and designed the experiments. ZL, WL, YX and WC performed the experiments. ZL analyzed the data. ZL, XT and RZ wrote the manuscript. All authors reviewed and approved the manuscript.
-
The authors declare that they have no conflict of interest.
-
The research was approved by the Ethics Committee at the First Affiliated Hospital of Guangzhou Medical University. All methods were performed in accordance with the relevant guidelines and regulations. Both informed and written consent was obtained from the participants who provided the samples.
















 DownLoad:
DownLoad: