HTML
-
Hepatitis B virus (HBV) belongs to the hepadnaviridae family and mainly infects hepatocytes, potentially causing acute or chronic hepatitis, cirrhosis and even hepatocellular carcinoma (HCC), and seriously threatening human health (Schweitzer et al. 2015). According to the World Health Organization (WHO) recently, approximately 240 million people worldwide suffer from chronic infections and approximately 680, 000 people die from liver disease caused by HBV (Ott et al. 2012). Presently, vaccination is the main measure to prevent chronic infections in China. More than 95% of the population is vaccinated after birth or during adolescence to effectively resist HBV infection (Seeger and Mason 2015; Hampel et al. 2016). Antiviral therapy remains the key method for HBV treatment, wherein the reduction of transaminase (especially alanine aminotransferase, ALT) levels and viral copies is the focus of current treatments for chronic infection (EASL clinical practice guidelines: Management of chronic hepatitis B virus infection 2012). Currently, there are no useful drugs worldwide that can eliminate HBV in vivo. Nucleos(t)ide analogues (NAs) and interferons (IFNs) are the most widely used anti-HBV drugs in the clinical environment, and can effectively reduce the incidence of HCC and improve the rates of long-term survival (Deres et al. 2003; Stanaway et al. 2016). It is well-known that RNA replication processes required for RNA virus replication and reverse transcription processes required for retroviral replication do not occur in human cells (Tong and Revill 2016); therefore, both replication processes must use their polymerase. NAs specifically target viral polymerases and selectively inhibit their replication. Commonly used Nucleos(t)ide analogs include lamivudine (LAM), adefovir dipivoxil (ADV), telbivudine (TBV), entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) (Marcellin et al. 2008; Liaw et al. 2009; Wu et al. 2014). The long-term use of NAs induces structural changes in viral polymerases to enhance their specificity. As a consequence, viruses in vivo can identify NAs and become drug-resistant mutants (Purdy et al. 2008; Lange et al. 2009; Lampertico et al. 2015). Pegylated interferons (PegIFNa) that are commonly used clinically, and have multiple functions such as immunomodulation, anti-tumor and anti-virus (Papatheodoridis et al. 2001). There is a significant decline in the incidence of liver cirrhosis, HCC and other related diseases after long-term use of IFNs in patients with chronic infections who are suitable for IFNs therapy (Niederau et al. 1996; Perrillo et al. 2015). According to several relevant scientific reports, more than 20% of patients treated with IFNs achieve the "ideal endpoints of therapy" (Brunetto et al. 2002). However, IFN therapy also has evident side effects; the most common appearance is poor tolerance, such as flu-like syndromes manifested as fever, headache, muscle aches, and limb weakness (van Zonneveld et al. 2004; Kwon and Lok 2011).
Therefore, the development of highly effective, safe, non-tolerant, and low-toxicity anti-HBV drugs is a significant challenge for all researchers and has important practical aspects. In this study, we used HepAD38 cells, a cell line that can activate high-efficiency HBV replication under the presence of tetracycline or its analogues (Ladner et al. 1997), to establish a high-throughput evaluation system in vitro, and to evaluate 796 compounds of the FDA-approved Drug Library. After the detection of HBV markers, HBsAg, HBeAg, and HBV DNA in the supernatant, arbidol hydrochloride, and antazoline hydrochloride were found to have a dosage-dependent inhibitory effect on HBV DNA. The EC50 of arbidol hydrochloride on HBV DNA in the supernatant was 4.321 μm/L, whereas that of antazoline hydrochloride was 2.910 μm/L showing higher significance. These findings provide new ideas for the screening and research of HBV agents, and make it possible to find effective drugs quickly and efficiently.
-
HepAD38 cell line, which has a tetracycline-responsive CMV-IE promoter located upstream part of the sequence, can replicate HBV in the absence of tetracycline (Tet) and its analogues in the growth medium. While in the presence of Tet, the cells are free from HBV due to the repression of pregenomic (pg) RNA synthesis. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS) (Gibco), 1% penicillin/streptomycin (P/S) (Invitrogen) and 2 μg/mL deoxytetracycline (DOX) (Schulze et al. 2010). Human hepatoma Huh7 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% P/S (Invitrogen) as described previously (Wu et al. 2019).
-
The FDA-approved Drug Library used in this study, which includes the anti-infection library and the GPCRG protein library, was purchased from MCE. The concentrations of the drugs of the library that were resuspended in dimethyl sulfoxide (DMSO) (Sigma) were 10 mmol/L. They were diluted to 10-100 μm/L when used. LAM, arbidol hydrochloride, and antazoline hydrochloride powder were purchased from TargetMol and diluted to 10 mmol/L. HepAD38 cells (DOX was removed 1 day before plating) were plated into 96-well collagen plates at 4 × 104 per well and incubated for 16 h. Drugs were diluted to 10 μm/L or 100 μm/L with DMEM and added to wells. To every plate, DMSO with medium was included for comparison as a control. After 72 h of incubation, the supernatants were renewed, and the compound was diluted to the same concentration. Supernatants were collected after 48 h.
-
For cell transfection, Huh7 cells were plated into 12-well plates at 4 × 105 cells per well a day before transfection. After approximately 16 h, the cell density was approximately 60%-80%, and the pHBV1.3-WT, which contains a 1.3-mer over-length HBV genome and was kindly supplied by Professor Dongliang Yang, was transfected into Huh7 cells according to the manufacturer's protocol using the Lipofectamine 2000 transfection reagent (Invitrogen).
-
The WST-1 solution was prepared based on the instructions of the WST-1 Cell Proliferation and Cytotoxicity Assay Kit (Beyotime). HepAD38 cells were plated in 96-well plates at 4 × 104 cells per well, and after 5 days of dosing treatment, culture supernatants were collected. WST-1 solution was diluted into 1 × using DMEM containing 10% FBS, and 1% P/S 100 μL 1 × solution was added to each well of the 96-well plates. After incubation for 1 h, the absorbance values (OD) at 450 nm and 630 nm were read with a microplate reader (BioTek).
-
Enzyme-linked immunosorbent assay (ELISA) was performed according to the recommendations of the manufacturer (KHB, Shanghai). The collected cell supernatants were diluted to a suitable concentration using PBS and the levels of HBeAg and HBsAg were measured. The OD values were read at 450 nm and 630 nm with an Epoch Microplate Spectrophotometer (BioTek) to export the statistics.
-
HepAD38 cells were cultured in 96-well plates for 5 days as described previously. A Hepatitis B Virus DNA Quantitative Fluorescence Diagnostic Kit (Shengxiang) was used to detect copies of HBV DNA in supernatants according to the recommendations of the manufacturer. After the treatment of Huh7 cells with drugs for 4 days, HBV DNA was extracted from cell culture supernatants using the QIAamp DNA Blood Mini Kit (QIAGEN). Realtime PCR, which was utilized to quantify progeny DNA, was performed on an ABI QuantSudio 6 Flex device.
-
HepAD38 cells and Huh7 cells were cultured in 12-well plates and treated with drugs as described above. Intracellular core-associated HBV DNA was extracted and applied to agarose gel electrophoresis using a previously reported method (Wu et al. 2010). The total RNA was collected using Trizol Reagent (Invitrogen) and electrophoresis was conducted in an RNA-free agarose gel (Ambion) according to the protocols of the manufacturer. After preparing of the HBV DNA probe with a High Prime DNA Labeling Kit (Roche), Southern and Northern blots were performed as described previously (Pei et al. 2014; Zhou et al. 2014) using a Northern Max Kit (Ambion) according to the recommendations of the manufacturer.
-
HepAD38 cells and Huh7 cells were cultured as described earlier. Intracellular protein samples were collected and separated by SDS-PAGE and then transferred to nitrocellulose filters (NC filters) (BIO-RAD) as previously described (Zhao et al. 2018). The primary antibodies used were mouse anti-b-actin monoclonal antibody (Santa Cruz) and rabbit anti-core polyclonal antibody (Gene). Horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno Research) and a chemiluminescence kit (Thermo Scientific) were used to bolt samples.
Cell Culture
FDA-Approved Drug Library and Treatment
Cell Transfection
Cytotoxicity Assay
Enzyme-Linked Immunosorbent Assays
Real-Time PCR
Southern Blot and Northern Blot
Western Blot
-
A concentration of 50% cytotoxicity (CC50) and the 50% effective concentration (EC50) were calculated using Graphpad Prism 5. The inhibitory potency and toxicity of the compounds were calculated based on at least three independent experiments. Data are presented as the mean ± standard error of the mean. The statistical significance of differences in multiple comparisons was deter-mined using the student's t-test. P < 0.05 was considered statistically significant.
-
HepAD38 cells were cultured in 96-well plates for 5 days as described previously. After collection of the supernatant, HBeAg, HBsAg, and HBV DNA were detected using specific kits as previously described. The initial concentrations of compounds from the anti-infection and the G Protein-Coupled Receptors/ G (GPCRG) protein libraries were 100 μm/L and 10 μm/L, respectively. These compounds were found to have no significant inhibitory effect on HBeAg and HBsAg in cell culture supernatants, but some of them showed noteworthy inhibitory effect on HBV DNA (Fig. 1A and 1B). Therefore, using WST-1 from the cytotoxicity assay kit and HBV DNA as the main indicators, compounds with low cytotoxicity and the inhibition of HBV DNA of more than 80% were selected. Hence, 68 compounds of the FDA-approved library were identified in HepAD38 cells (Fig. 1C and 1D). The compounds were diluted to 100 μm/L or 30 μm/L with DMEM and three replicate wells were set with a three-fold concentration gradient. After 5 days of incubation, HBeAg, HBsAg, and HBV DNA in supernatants were detected using kits as previously described. The cytotoxicity of the compounds was investigated using WST-1 kits. Results showed that arbidol hydrochloride (Fig. 1E) of the anti-infection library and antazoline hydrochloride (Fig. 1F) of the GPCRG library exhibited no significant cytotoxicity at a concentration of 10 μm/L and had a dose-dependent inhibition of HBV DNA in the supernatant.
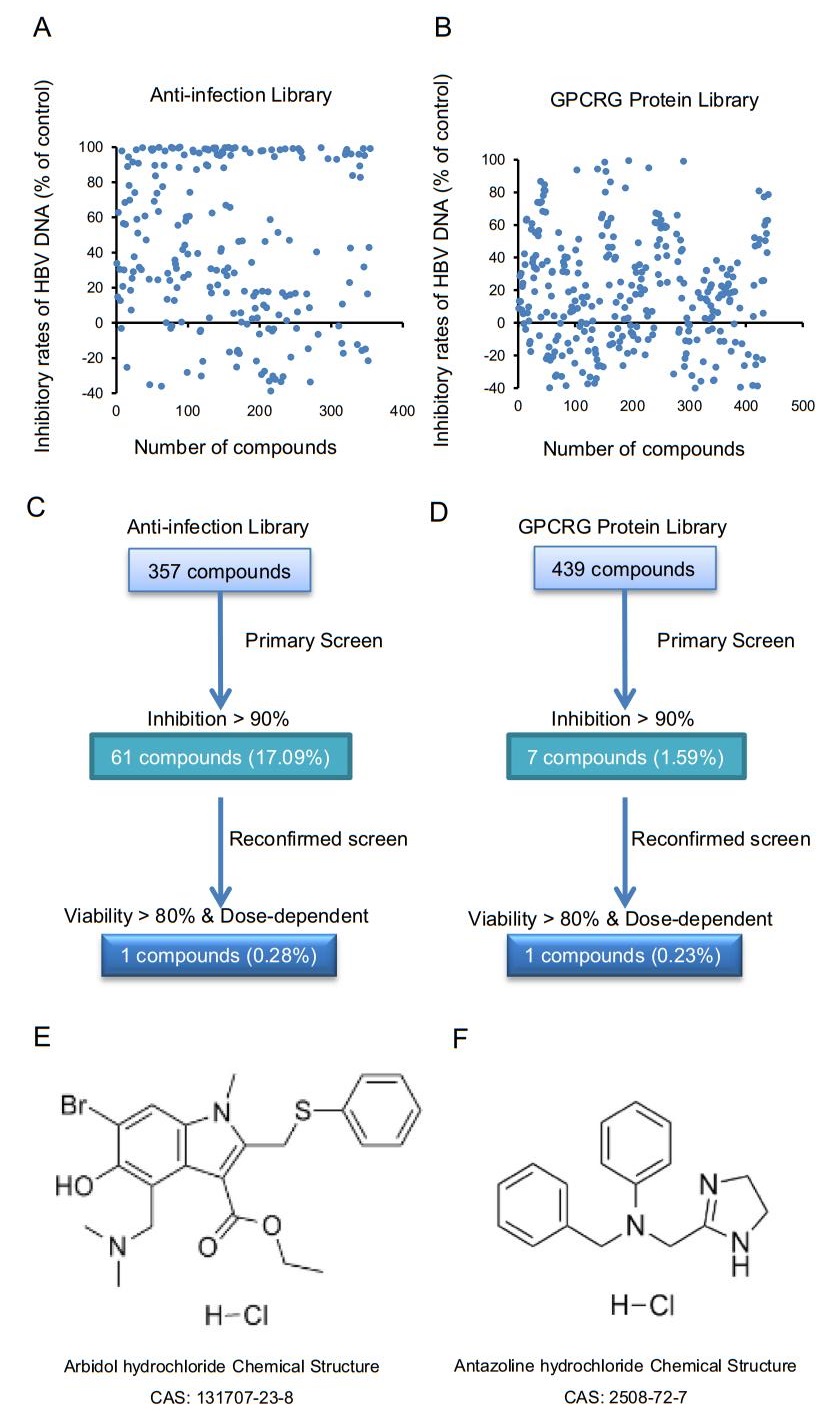
Figure 1. Inhibitory effects of compounds in FDA-approved library. A Inhibitory effects of 357 compounds of antiinfection library on HBV DNA in the supernatant, wherein each compound represented a concentration of 100 μm/L. B Inhibitory effects of 439 compounds in the GPCRG library on HBV DNA in the supernatant, wherein each compound represented concentration of 10 μm/L. C Flow chart of high-throughput screening of anti-infection library. Total of 61 compounds with inhibitory rate greater than 90% at concentration of 100 μm/L were selected from 357 compounds. After reconfirmation, compound with a viability of more than 80% at 10 μm/L and dose-dependent inhibition of HBV DNA, arbidol hydrochloride, was selected as shown in (E). D Flow chart of high-throughput evaluation of GPCRG library. Total of 7 compounds with inhibitory rate greater than 90% at dose of 10μm/L were selected from 439 compounds. After reevaluation, compound with a viability of more than 80% at dose of 10 μm/L and dose-dependent inhibition of HBV DNA, antazoline hydrochloride, was selected as shown in (F).
-
To test the pharmacodynamics of arbidol hydrochloride, HepAD38 cells were plated into 96-well collagen plates at 4 × 104 per well and incubated for 16 h. Arbidol hydrochloride was diluted to 100 μm/L with a medium as described above, and after 5 days of dosing, cell culture supernatants were collected. The toxicity of arbidol hydrochloride to cells was detected using the WST-1 kit, and an inhibitory effect on HBeAg, HBsAg, and HBV DNA in the culture supernatant was detected by ELISA and real-time PCR, respectively (Fig. 2). Intracellular HBV core-associated DNA was collected, and its level of replication was examined by Southern blot. We found that CC50 of arbidol hydrochloride was greater than 10 μm/L (Fig. 2A), and EC50 of arbidol hydrochloride on HBV DNA in the supernatants was 4.321 μm/L (Fig. 2B). Results of Southern blot showed that the compound also inhibited intracellular HBV core-associated DNA in a dose-dependent manner (Fig. 2C). However, this compound had no obvious inhibitory effect on HBsAg in cell culture supernatants (Fig. 2D) and had a weak inhibitory effect on HBeAg (Fig. 2E). The inhibitory effect became weaker as the concentration decreased.
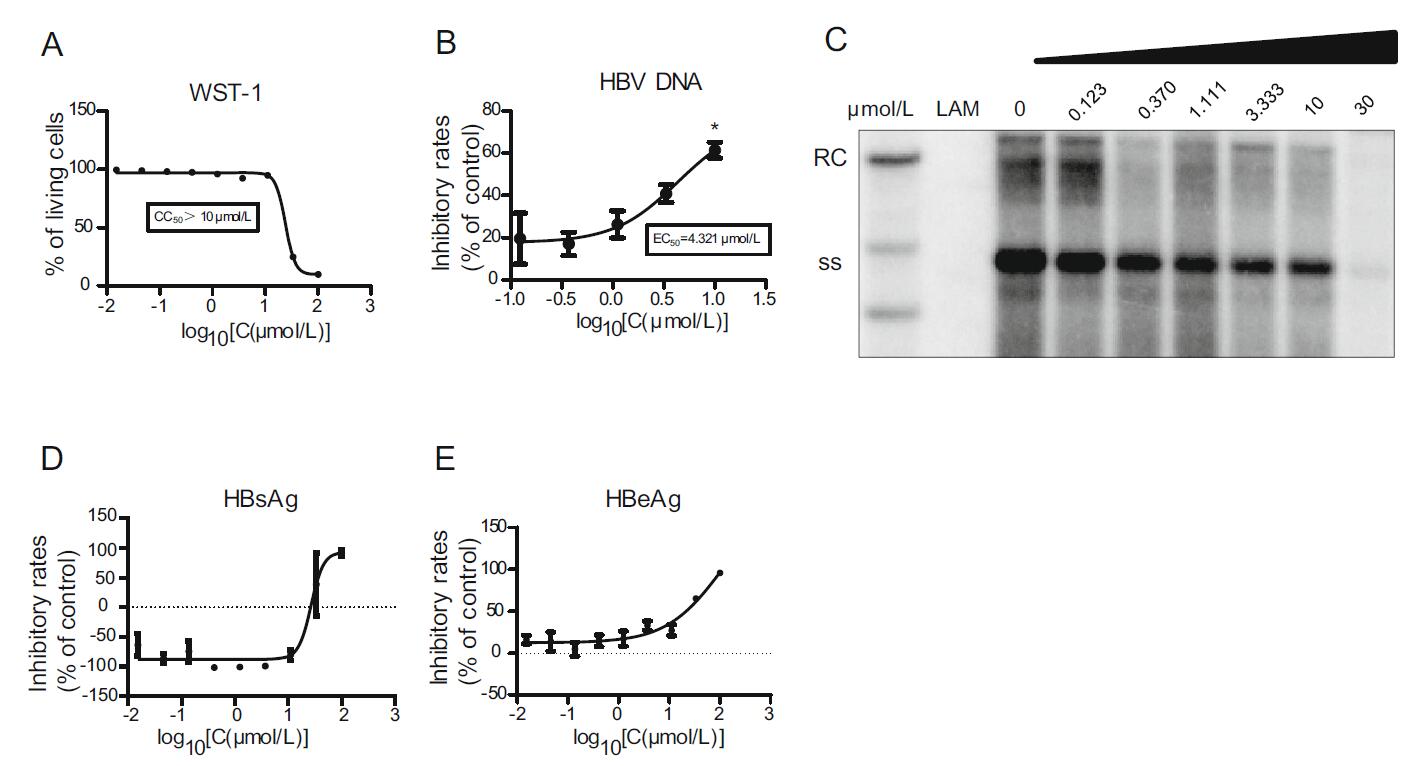
Figure 2. Pharmacodynamics of arbidol hydrochloride. HepAD38 cells were plated into 96-well collagen plates, and arbidol hydrochloride was diluted to 100 μm/L with the medium and added to the plates. After 5 days of culture, supernatants were collected, and cytotoxicity of arbidol hydrochloride was detected using WST-1 kit (A). ELISA and real-time PCR were used to detect inhibitory rates of arbidol hydrochloride on HBeAg (E), HBsAg (D), and HBV DNA (B) in the culture supernatant. Southern blot was used to detect levels of replication of HBV core-associated DNA (C). Data shown here are representative of at least three independent experiments. A two-tailed t-test was used to determine differences statistically (*P < 0.05, compared to the group with drug treatment at the lowest concentration). There were no significant differences for the data sets in D and E. RC, relaxed circular DNA; ss, single-stranded DNA.
HepAD38 cells were plated and incubated as described previously to detect the inhibitory effect of antazoline hydrochloride. The results indicated that CC50 of antazoline hydrochloride was greater than 30 μm/L (Fig. 3A), and EC50 of antazoline hydrochloride on HBV DNA in supernatant was 2.910 μm/L (Fig. 3B). There were no significant inhibitory effects perceived on HBeAg and HBsAg in the supernatant (Fig. 3C and 3D).
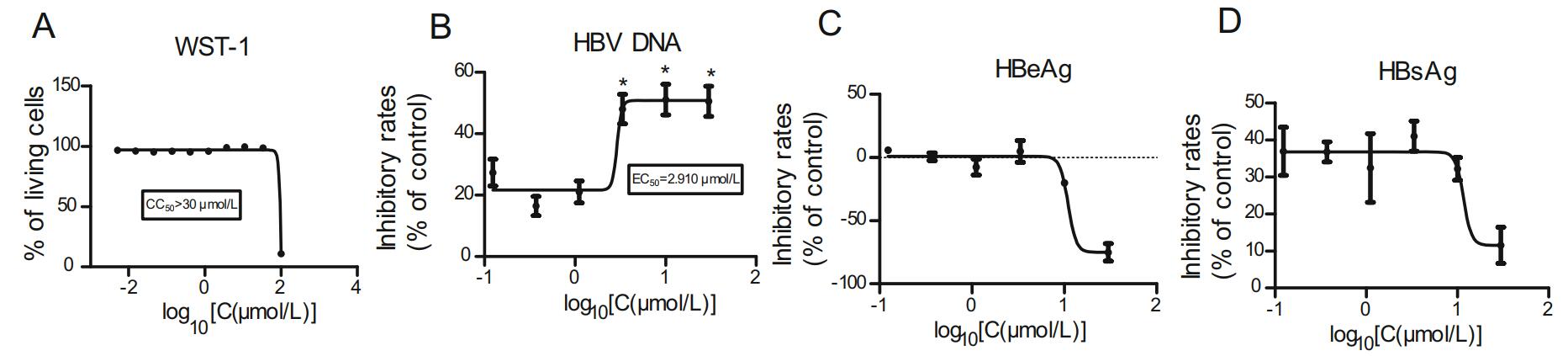
Figure 3. Pharmacodynamics of antazoline hydrochloride. HepAD38 cells were plated into 96-well collagen plates, and antazoline hydrochloride was diluted to 100 μm/L with the medium and added to the plates. After 5 days of culture, supernatants were collected, and cytotoxicity was detected using WST-1 kit to obtain CC50 (A) of antazoline hydrochloride. ELISA and real-time PCR were used to detect the inhibitory rates of antazoline hydrochloride on HBeAg (C), HBsAg (D), and HBV DNA (B) in the culture supernatant. Data shown here are representative of at least three independent experiments. A two-tailed t-test was used to determine differences statistically (*P < 0.05, compared to the group with drug treatment at the lowest concentration). For the other data sets, there were no significant differences.
A comprehensive comparison of two compounds, arbidol hydrochloride and antazoline hydrochloride, was conducted. Arbidol hydrochloride is a broad-spectrum antiviral drug that has recently been reported that it has an inhibitory effect on the dimerization of HBV core protein (Wei et al. 2018). Antazoline hydrochloride is a first-generation antihistamine with anticholinergic properties; it can be used as eye drops and to relieve nasal congestion (Farkowski et al. 2019). However, an inhibitory effect on HBV has not been reported yet; thus, antazoline hydrochloride was selected as a candidate.
-
To further explore the inhibitory mechanism of antazoline hydrochloride on HBV, HepAD38 cells were plated into 12-well plates and incubated as described earlier. This was found to have no significant inhibitory effect on intracellular HBV core-associated DNA, RNA, and HBcAg (Fig. 4). In other words, the dosage-dependence of antazoline hydrochloride on HBV indicators was not observed in HepAD38 cells.
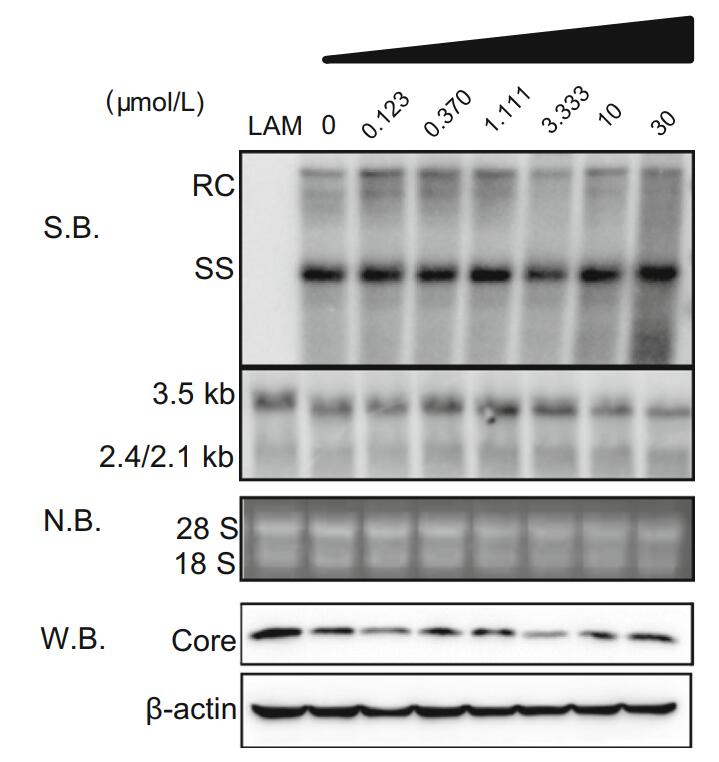
Figure 4. Effects of antazoline hydrochloride on intracellular protein, HBV DNA, and RNA. After HepAD38 cells were treated with antazoline hydrochloride for 120 h, intracellular protein samples, HBV DNA and total RNA were collected. Western blot was used to detect effect of antazoline hydrochloride on expression of core protein. Southern and northern blots were used to detect effect of antazoline hydrochloride on HBV transcription and replication intermediates. RC, relaxed circular DNA; ss, single-stranded DNA; S.B., Southern blot; N.B., Northern blot; W.B., Western blot.
-
To further study the inhibitory effect of antazoline hydrochloride on the HBV DNA, we constructed a transfection model using Huh7 cells. After 96 h of culture, the supernatants were collected. HBV DNA in cell supernatants was extracted using kits and the DNA copy number was detected by real-time PCR. We found that LAM also effectively inhibited HBV DNA in supernatants in this system, and the EC50 of LAM on HBV DNA in the supernatant was 131.0 nmol/L at a concentration of 10 μm/L (Fig. 5A and 5B). In this model, antazoline hydrochloride also had a significant inhibitory effect on HBV DNA in supernatants in a dose-dependent manner. The EC50 in the transfection system was 2.349 μm/L (Fig. 5C) while antazoline hydrochloride had no significant inhibitory effect on intracellular HBV core-associated DNA and HBcAg protein, which was consistent with the results obtained in HepAD38 cells (Fig. 5D). Based on the above results, we hypothesized that antazoline hydrochloride acts on the release phase of the HBV life cycle and inhibits copies of HBV in cell supernatants by preventing the release of mature HBV particles.
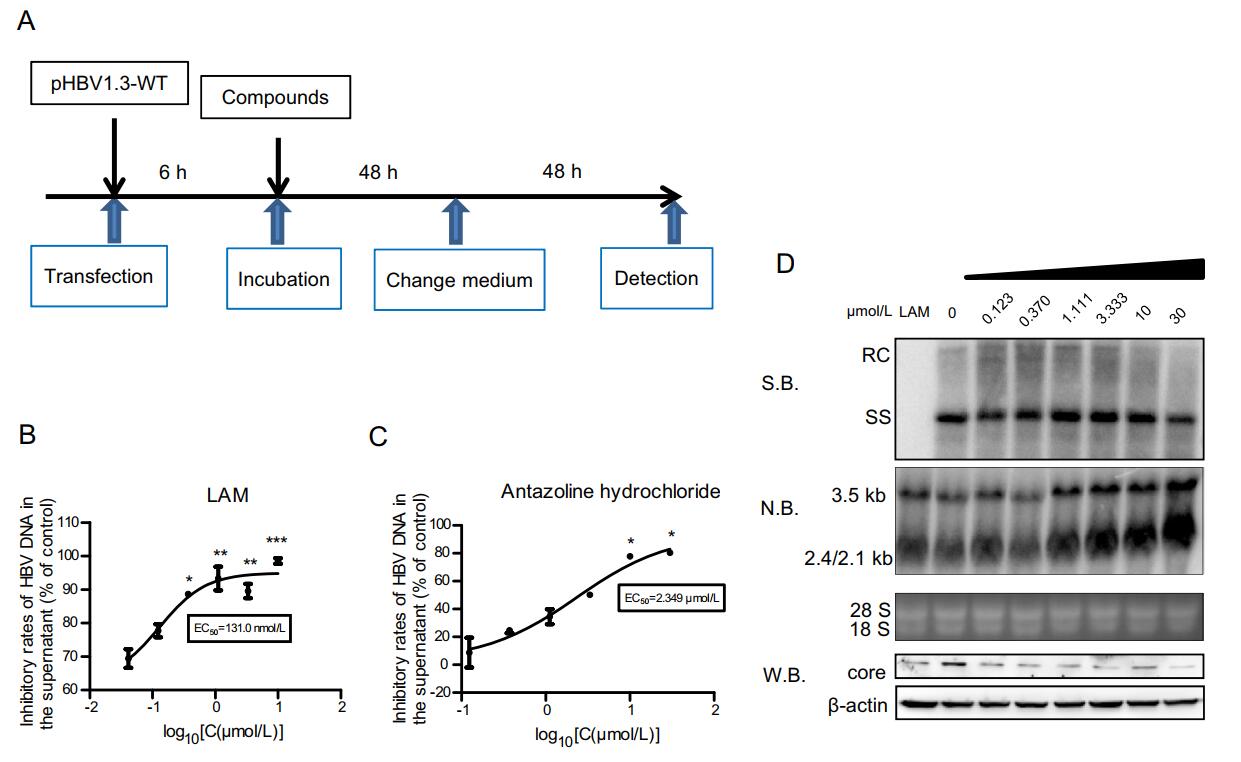
Figure 5. Inhibitory effect of antazoline hydrochloride in transfection system. A Huh7 cells were plated into 12-well plates and incubated for 16 h. pHBV1.3-WT plasmid was transfected into the cells. After 48 h of incubation, supernatants were changed and compounds were diluted to same concentration. Supernatants were collected after 48 h. B LAM was diluted in concentration gradients of 30 μm/L, 10 μm/L, and 3.33 μm/L. After dosing and culture as described above, HBV DNA in the cell supernatant was extracted by kits and detected using realtime PCR. C Antazoline hydrochloride was prepared at 30 μm/L, 10 μm/L, 3.33 μm/L, 1.11 μm/L, 0.370 μm/L, and 0.123 μm/L. After 96 h of culture, HBV DNA in the cell supernatant was extracted using kits and detected by real-time PCR. EC50 for antazoline hydrochloride in HBV DNA is shown. D After 5 days with dosing of antazoline hydrochloride, intracellular protein, HBV core-associated DNA and RNA were collected and detected by Western blot, Southern blot and Northern blot. Data shown here are representative of at least three independent experiments. A two-tailed t-test was used to determine differences statistically (*P < 0.05, **P < 0.01, and ***P < 0.001, compared to the group with drug treatment at the lowest concentration). RC, relaxed circular DNA; ss, single-stranded DNA; S.B., Southern blot; N.B., Northern blot; W.B., Western blot.
Inhibitory Effects of Compounds in FDA-Approved Library
Pharmacodynamics of Arbidol Hydrochloride and Antazoline Hydrochloride
Inhibitory Effects of Antazoline Hydrochloride on Intracellular Protein, HBV DNA, and RNA
Inhibitory Effect of Antazoline Hydrochloride in Transfection System
-
For a long time, drug development of HBV has been one of the main areas of focus for the government and research institutions, with an aim that drugs for HBV with high efficiency and low toxicity become available. It is well known that one of the difficulties in the development of anti-HBV drugs is the lack of a reliable and stable com-pound screening system. In recent years, the establishment of human primary hepatocyte (PHH) infectious model in vitro has enabled most compounds to be effectively judged, which created opportunities that facilitated their application in clinical research. However, the key to infectious system, PHH, is not easy to be obtained, and culture conditions are demanding and costly being unsuitable for high-throughput screening. This made the selection of HepG2.2.15 cells and HepAD38 cells reasonable for the study. During the early stage of this project, we attempted to establish a high-throughput screening system using HepG2.2.15 due to its ability to stably express all viral markers of HBV; including HBsAg, HBeAg, and HBV DNA. Besides, intact Dane particles were also observed in supernatants of HepG2.2.15, and several of viral replication intermediates can be detected. However, the cell line was found out to only gradually reflect the effects of drugs during the application of longrange (more than 10 days) cultures, while it was also perceived that cell state of the laboratory cannot support such a long-term culture. In addition, there was an attempt to establish a Huh7DhNTCP evaluation model, which was inserted into a gene fragment of sodium taurocholate co-transporting polypeptide (NTCP) into the DMSO-tolerant Huh7D genome to stably express it in the cell. This supported the infectious phases of HBV. However, during the experiment the inhibitory effect of compounds on HBV was observed in culture for more than 12 days, although cells were in poor condition after 10 days of cultivation, and many dead cells were observed under the microscope. Thus, the cell line deemed unsuitable for establishing a screening system. Therefore, HepAD38 cells were eventually selected.
HepAD38 cells were constructed by inserting the 1.1-fold genome of HBV into HBV sequences. The exogenous promoter CMV-IE located upstream of the sequences enhances the transcription of the HBV genome and is regulated by tetracycline and its analogues. When tetracycline or its analogues are added, the CMV promoter is turned off. After the removal of tetracycline, HBV markers are soon found in the culture supernatant. After further exploration, it was found that the HBV markers produced by HepAD38 could be observed after 5 days of dosing, as well as that inhibitory effects of compounds could be detected and the cultivation time was greatly reduced for the cell line.
In this study, the toxicity and inhibitory effect of compounds on HBV DNA in supernatants were used as main indicators, and the compounds of FDA-approved Drug Library were screened by constructing a high-throughput evaluation system in vitro. Finally, two compounds, arbidol hydrochloride and antazoline hydrochloride, were selected for further research. Arbidol hydrochloride is a broadspectrum antiviral drug that inhibits the entry of enveloped viruses by blocking the fusion of the virus with the membranes of the host cell. Recently, it has been reported that this compound exerts an inhibitory effect on HBV as it takes part in the formation process of core protein nucleocapsids; therefore, we did not conduct mechanistic research on arbidol hydrochloride. Meanwhile, antazoline hydrochloride is the first-generational antihistamine with anticholinergic properties that help relieve nasal congestion and are used in eye drops. However, its inhibitory effect on HBV has not been reported so far, therefore it can be used as a candidate drug and as well as a target for in-depth research of the mechanism. Through our investigation, it was found that antazoline hydrochloride showed good inhibitory effect on HBV DNA in the supernatant of HepAD38 and Huh7 cells with EC50 value of 2.910 μm/L and 2.349 μm/L, respectively. After preliminary exploration of the mechanism of antazoline hydrochloride, it was found that the compound had no significant inhibitory effect on intracellular HBV core-associated DNA, RNA, and HBcAg. This suggests that future work should be focus on the mechanisms related to the release phase of the HBV life cycle, and on the verification of this hypothesis in different cell systems. The reuse of antazoline hydrochloride can greatly shorten the entry time to clinical trials and reduce the requirements for clinical verification of toxic and side effects. This not only saves a significant amount of manpower and material cost, but also has certain reference value for the development of different target drugs of HBV in the future.
-
We would like to thank members of Professor Xinwen Chen's Laboratory, Professor Dongliang Yang, and Dr. Kaitao Zhao. This work was supported by grants from the National Nature Science Foundation of China (31770180).
-
JL and YH performed the research and analyzed the data. JL, YH, YY, YZ, QH, and CL prepared the figures and tables. CW, RP, XC, and YG wrote the manuscript. XH, YZ, CW, YW, and YG contributed reagents/analysis tools/materials. RP, CW and XC designed and supervised the overall study. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: