HTML
-
The innate immunity presents one of the first lines of defense against disease-causing pathogens, including viruses (Wells and Coyne 2018). The innate response is initiated by the rapid and efficient detection of pathogenassociated molecular patterns (PAMPs) via pattern recognition receptors (PRRs). During virus infections, viral nucleic acids, i.e. viral DNA and/or RNA, are the major PAMPs that trigger an innate response. The activation of PRRs trigger signaling cascades that can lead to the activation of immune and non-immune cells, thereby initiating antiviral responses that restrict and interfere with virus replication and further virus spread. A significant antiviral response elicited upon sensing of viruses is the interferon (IFN) response. IFN can be subdivided into type Ⅰ IFN (mainly IFN-α and IFN-β as well as several other IFN subtypes), type Ⅱ IFN (IFN-γ) and type Ⅲ IFN (IFN-λ). Type Ⅰ and Ⅲ IFN belong to the innate immune response and are mainly produced by virus-infected cells and plasmacytoid dendritic cells (pDC), whereas type Ⅱ IFN is typically categorized as part of the adaptive immune response and is produced mainly by T cells (e.g. Th1 cells and cytotoxic T lymphocytes) but also natural killer cells (NK cells) (Huang et al. 2014).
The type Ⅲ IFN or interferon (IFN)-k system is the most recently identified type of IFN, as it was first described in 2003 (Kotenko et al. 2003; Sheppard et al. 2003). Four human cytokines, IFN-λ1 (also called IL29), IFN-λ2 (also called IL28A), IFN-λ3 (also called IL28B) and IFN-λ4 were identified, with IFN-λ1–3 sharing high amino acid sequence homology, whereas IFN-λ4 is more divergent with only 29% amino acid similarity to IFN-λ3 (Onabajo et al. 2019). IFN-λ4 was only discovered in 2013 and is the most enigmatic IFN (Prokunina-Olsson et al. 2013). In human, it can be expressed only in individuals carrying the DG allele of a dinucleotide genetic variant rs3687234815-TT/DG. Curiously, whereas IFN-λ4 displays potent antiviral activity in vitro against different viruses including hepatitis C virus (HCV), it is poorly secreted and is associated with reduced viral clearance of HCV from the liver (Prokunina-Olsson et al. 2013). IFNk4 is not found in mice and rats (and nonmammals) although most higher mammals appear to encode biologically active homologs of IFN-λ4, including e.g. chimpanzee, orangutan, cynomolgus, marmoset, dog, pig and bat (Paquin et al. 2016). Mice possess only two functional type Ⅲ IFNs, IFN-λ2 and IFN-λ3, as in this species IFNk1 is a pseudogene (Zanoni et al. 2017). In general, the IFN-λ antiviral system is highly conserved in evolution and can be traced back at least to birds as the chicken genome encodes a functional IFN-λ gene that shows some structural homology to human IFN-λ3 (Karpala et al. 2008; Donnelly and Kotenko 2010; Arslan et al. 2019).
Early studies showed that all IFN-λs bind to a heterodimeric IFN-λ receptor composed of the IFN-λ receptor 1 (IFNLR1 or IL28RA) chain and the IL-10 receptor subunitb (IL-10RB) chain (Fig. 1). Although type Ⅲ IFNs show little homology to type Ⅰ IFNs and despite the fact that the type Ⅲ IFN receptor is entirely different from the type Ⅰ IFN receptor (consisting of the IFNAR1 and IFNAR2 receptor chains), intracellular signaling initiated by binding of type Ⅰ or type Ⅲ IFN to their corresponding receptor is virtually identical. Indeed, in both cases, upon ligand binding, the receptor-associated Janus kinase family (JAK family) members JAK1 and tyrosine kinase 2 (TYK2) undergo phosphorylation and activation. However, more recently, it was shown that JAK2 may also be phosphorylated by type Ⅲ IFNs, but not type Ⅰ IFNs (Odendall et al. 2014). Both for type Ⅰ and type Ⅲ IFN, JAK family activation then leads to tyrosine phosphorylation of receptor-associated signal transducer and activator of transcription (STAT) proteins STAT1 and STAT2, resulting in the formation of STAT1–STAT2 heterodimers. In turn, STAT1–STAT2 heterodimers associate with interferon regulatory factor 9 (IRF9) and this trimeric signaling complex consisting of STAT1, STAT2 and IRF9—which is known as interferon-stimulated gene factor 3 (ISGF3)— then moves to the nucleus and binds to interferon-stimulated response element (ISRE) motifs in the promoter regions of interferon-stimulated genes (ISGs), thereby triggering enhanced transcriptional activity of these genes (Zhou et al. 2007) (Fig. 1).
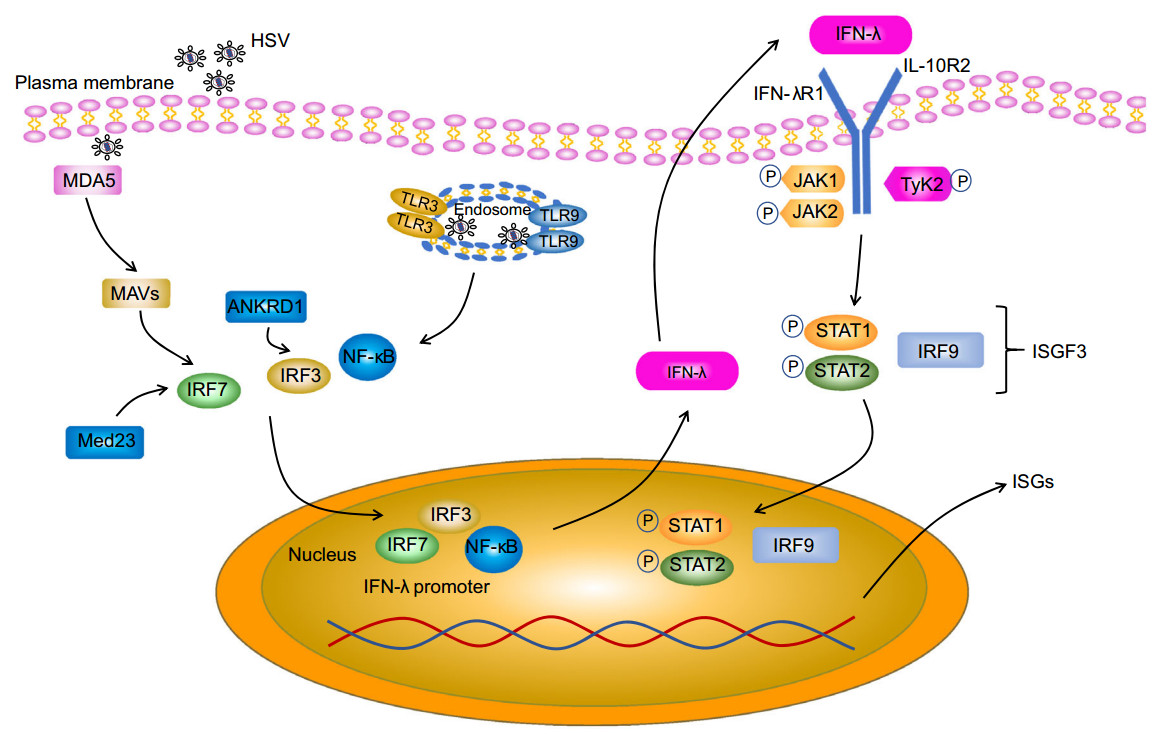
Figure 1. Pathway of Type Ⅲ IFN induction and signaling, and interaction of type Ⅲ IFN with HSV. HSV molecular patterns are recognized by TLR3 and TLR9 in the endosome and/or MDA5 in the cytoplasm, leading to the activation of the NF-κB, IRF3 and IRF7 transcription factors and their subsequent translocation to the nucleus where they stimulate IFN-λ gene transcription. Med23 and ANKRD1 are direct targets of IRF7 and IRF3 respectively and upregulate type Ⅲ IFN expression. Secreted IFN-λ binds to a heterodimeric receptor composed of the IFNLR1 and IL-10RB receptor chains. IFN-λ binding leads to activation of JAK1/TYK2, which in turn leads to tyrosine phosphorylation of STAT1 and STAT2, resulting in the formation of STAT1–STAT2 heterodimers. Subsequently, STAT1– STAT2 heterodimers associate with IRF9, forming the ISGF3 complex, which then moves to the nucleus and triggers expression of interferon-stimulated genes (ISGs).
Although type Ⅰ and type Ⅲ IFN both belong to the innate immune response and display apparent similarities in signaling pathway and biological functions, both types of IFN display important differences that affect their biological functions. Whereas the receptor for type Ⅰ IFNs is expressed broadly in virtually every cell type, the expression of the IFNLR1 receptor is much more restricted, mainly to epithelial cells that line mucosal surfaces. In addition, whereas most if not all cells are able to express type Ⅰ IFN, particularly when they are infected with a virus, only specific cell subsets, again particularly epithelial cells, are able to express type Ⅲ IFN. Hence, it is believed that type Ⅲ IFN is of paramount importance as a first line and local innate defense against viral pathogens that interact with mucosal barriers of the intestinal, the respiratory and genital tracts, whereas type Ⅰ IFN may in fact represent a second line defense in the context of these types of pathogens, with more systemic effects and thereby more widespread and sometimes potentially harmful consequences (Ye et al. 2019). Although it is not entirely clear how IFNLR1 expression is largely restricted to cells of epithelial origin, there are indications that cell typedependent epigenetic changes of the IFNLR1 gene, including DNA methylation and histone modifications, may be involved (Ding et al. 2014). Indeed, DNA methyltransferase inhibitors and histone deacetylase (HDAC) inhibitors have been reported to release silencing of the IFNLR1 gene, increasing receptor expression and restoring IFN-λ sensitivity in previously non-responsive cells, thereby enhancing protection against viral pathogens including herpes simplex virus 1 (HSV1) (Ding et al. 2014).
Herpesviruses are a large family of double-stranded viruses. Virions consist of a linear dsDNA genome wrapped in a highly stable icosahedral capsid, surrounded by a partly unstructured protein layer called the tegument, and the outer lipid layer membrane envelope, which contains multiple viral (glyco) proteins. The herpesvirus family is divided into three subfamilies, the alphaherpesvirinae, betaherpesvirinae and gammaherpesvirinae, based on sequence homology and biological properties (Arvin et al. 2007). Alphaherpesviruses represent the largest subfamily of the herpesviruses and include a wide array of pathogens of man and animal including herpes simplex viruses 1 and 2 (HSV-1 and HSV- 2), varicella zoster virus (VZV), pseudorabies virus (PRV) in pigs, bovine herpesvirus 1 (BoHV-1) in cattle, equine herpesvirus 1 (EHV1) in horses, and Marek's disease virus (MDV) and duck plague virus (DPV) in birds. Betaherpesviruses include e.g. human cytomegalovirus (HCMV), murine cytomegalovirus (MCMV), and human herpes viruses 6A, 6B and 7 (HHV-6A, -6B and -7), whereas members of the gammaherpesviruses include e.g. human pathogens Epstein-Barr virus (EBV) and Kaposi's sarcomaassociated herpesvirus (KSHV), alcelaphine gammaherpesvirus 1 (ALHV-1) in wildebeest and cattle and murid herpesvirus 4 (MuHV-4) in mice (Albà et al. 2001).
Herpesviruses enter their host via mucosal surfaces, typically of the respiratory and/or genital tract, and invariably cause lifelong latent infections in their natural host. Primary lytic replication of these viruses typically occurs in epithelial cells of the mucosal membrane. The site of latent infection depends on the type of virus, with most alphaherpesviruses residing latently in sensory neurons whereas beta- and gammaherpesviruses establish latency in different immune cell populations. Stress, immune suppression and other triggers may lead to (periodic) reactivation of herpesviruses from latency, which may result in recurrent virus spread within the host and to other hosts and typically again involves virus replication at mucosal sites (Grinde 2013). Hence, herpesviruses likely interact with the type Ⅲ IFN system at several important stages in their replication, including during host entry and spread to other hosts. Despite the supposedly intimate and complex interaction between herpesviruses and the type Ⅲ IFN system, relatively little is known about this research field. In this review, we outline what is currently known about the interplay between herpesviruses and type Ⅲ IFN, where most information thus far has been derived from studies on HSV-1 and HSV-2. These viruses mainly cause cold sores (typically HSV-1) and genital herpes (typically HSV-2) but in rare cases may also cause more aggravated disease, including infectious blindness and life-threatening encephalitis (Grinde 2013).
-
In the context of HSV-1 infection, a vast amount of studies have shown that genetic polymorphisms that negatively affect a Toll-like receptor 3 (TLR3)-UNC-93B-dependent axis that leads to type Ⅰ and type Ⅲ IFN production are associated with life-threatening herpes simplex encephalitis (HSE) (Zhang et al. 2013). This signaling axis is essential to confer protective immunity to HSV-1 in the CNS during the course of primary infection in childhood.
In 2009, a single nucleotide polymorphism (SNP) was detected in the promoter region of human IFN-λ3 (rs12979860) which was associated with efficiency of clearance of hepatitis C virus (HCV), where the C/C genotype showed stronger virus clearance compared to C/T or T/T genotypes (Thomas et al. 2009). The increased clearance of HCV was later associated with increased serum levels of IFN-λ in patients with C/C genotype (Langhans et al. 2011). Interestingly and in line with this, in an Italian cohort of patients, the T/T genotype was also associated with increased disease severity of HSV1 herpes labialis and severe herpes labialis was associated with lower levels of serum IFN-λ (Pica et al. 2010; Griffiths et al. 2013). This polymorphism was also correlated to HSV seropositivity and IFN-λ serum levels in healthy patients and patients with Alzheimer's disease (AD) and mild cognitive impairment (MCI). IFN-λ serum concentrations were increased in AD and MCI patients carrying the T allele compared to healthy controls, and AD patients with the C/C genotype showed the highest anti-HSV1 serum antibody levels, further suggesting that this IFN-λ3 polymorphism modulates the immune response against HSV-1 (Costa et al. 2017). The latter study also is in line with suggestions that HSV-1 triggers a potent but ineffective IFN-λ response in Alzheimer's disease (and Parkinson's disease) patients (La Rosa et al. 2019).
Curiously, studies of this SNP in the context of HCMV replication in solid organ transplant recipients showed an opposite trend, with the T/T genotype associated with reduced HCMV replication (Egli et al. 2014; FernándezRuiz et al. 2015). Likewise, the same minor T/T allele was found to be associated with significantly shorter episodes of active HCMV infection in a cohort of haematopoietic stem cell recipients (Bravo et al. 2014). Follow-up in vitro investigation showed that human foreskin fibroblasts (HFF) carrying the minor T/T genotype displayed 3-log reduced virus titers upon HCMV infection, which was associated with increased IFNα and ISG but reduced IFN-λ mRNA levels (Egli et al. 2014). In addition to this apparent IFN-λinterference with IFNα-mediated control of HCMV, increased IFN-λ3 levels have been hypothesized to interfere with the development of an efficient HCMV-specific adaptive response, e.g. by hindering the priming of HCMV-specific T cells and/or by increasing levels of circulating regulatory T cells (Bravo et al. 2014; Egli et al. 2014). As such, although IFN-λ has been shown to display antiviral activity against HCMV in vitro (Brand et al. 2005), IFN-λ3 appears to suppress immune-mediated control of HCMV replication, at least in the context of transplant management.
In 2013, a dinucleotide polymorphism upstream of the human IFN-λ3 gene was identified (rs368234815, TT versus DG) that correlated very strongly with HCV clearance and at the same time led to the discovery of IFN-λ4 (Prokunina-Olsson et al. 2013). It was found that only individuals with the DG genotype could produce IFN-λ4, and that the DG genotype is strongly associated with impaired spontaneous HCV clearance (Prokunina-Olsson et al. 2013). Interestingly, the same minor DG/DG genotype was also significantly associated with increased occurrence of HCMV-caused renitis in a cohort of HIVpositive individuals (Bibert et al. 2014). In line with this, in a large cohort study of solid organ transplant recipients, it was found that patients carrying the minor DG/DG genotype showed increased HCMV replication, specifically in the group of patients that did not receive antiviral prophylaxis (Manuel et al. 2015). IFN-λ4 has been shown to interfere with the antiviral effects of IFNα via e.g. increased induction of USP18 and suppressor of cytokine signaling (SOCS1) (Fan et al. 2016; Blumer et al. 2017), which may aid to explain why IFN-λ4 expression may be associated with reduced control of virus infections. A large study investigating the impact of the same dinucleotide polymorphism on HSV-related outcome did not find any significant correlation (Kuhs et al. 2015).
-
The activation of the first phase of the type Ⅲ (or type Ⅰ) IFN response is dependent on the ability of PRRs to recognize PAMPs in the infected cells. Throughout the viral life cycle, several PRRs have been found that can recognize herpesviruses at different stages of their replication cycle. For HSV, some of these, like TLR2, TLR4, and HVEM, recognize viral envelope glycoproteins that are involved in virus attachment to and entry in the host cell. Others recognize HSV DNA, including TLR9 in endosomes, cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) and DNA dependent activator of IFN-regulatory factors (DAI) in the cytoplasm and IFNγ inducible protein 16 (IFI16) mostly in the nucleus. In addition, melanoma differentiation-associated protein 5 (MDA5), retinoic acid-inducible gene I (RIG-I), and protein kinase RNA-activated (PKR) recognize viral dsRNA that is produced in the cytoplasm (Danastas et al. 2020). Whereas most of these sensors have been associated with HSV-induced type Ⅰ IFN production, only some PRRs (TLR3, TLR9, RIG-I and MDA5) thus far have been linked to HSV-induced type Ⅲ IFN production (Fig. 1), as explained below.
-
TLRs are the first identified and best characterized PRRs, and all 10 TLRs members in human, except TLR3, trigger MyD88-dependent signaling, resulting in the translocation of NF-κB into the nucleus and various inflammatory responses. TLR7/8/9 also cause the translocation of interferon regulatory factor 7 (IRF7) into the nucleus via a MyD88-dependent pathway, which leads to type Ⅰ interferon responses. TLR3 utilizes TRIF instead of MyD88 to cause the translocation of IRF-3 into the nucleus, again ultimately resulting in the production of type Ⅰ interferons along with the activation of NF-κB (Jahanban-Esfahlan et al. 2019). Although TLR2, TLR3, TLR4 and TLR9 all have been identified to recognize HSV infection resulting in the production of type Ⅰ IFNs (Su et al. 2016), only TLR3 and TLR9 have been reported to trigger type Ⅲ IFN expression upon HSV infection thus far.
TLR-9 recognizes unmethylated CpG motifs in DNA, which are abundant in the HSV genome. TLR9-dependent type Ⅲ IFNs production was demonstrated both in vivo and in vitro (Yin et al. 2012). In an initial in vitro study on HSV-2, plasmacytoid dendritic cells (pDCs) and conventional DC (cDCs) were isolated from parental C57BL/6 and TLR9 -/- mice and incubated with HSV-2. The expression of IFN-α, IFN-β and IFN-λ in pDCs from C57BL/6 was found to be significantly higher than the corresponding response in pDC from TLR9 -/- mice. However, cDCs from both mouse strains showed comparable type Ⅰ and Ⅲ IFN production, suggesting that production of innate IFNs may not only require TLR9 in response to HSV-2 infection. In an in vivo experiment, IFNAR -/-, IL-28RA -/- mice and WT mice were treated with a TLR9 agonist (CpG ODN 1826) before vaginal infection with HSV-2. Viral titers were markedly reduced in vaginal washes from wild-type mice compared to those of IL-28RA -/- mice and IFNAR -/- mice (Ank et al. 2008). In a similar in vivo study, which in addition to this TLR9 agonist also assessed agonists of cytoplasmic sensors RIG-like receptors (RLR) and cytosolic DNA receptors (DNAR), it was found that although all three types of sensors depend on type Ⅰ IFN signaling to evoke antiviral activity against genital HSV-2 infection, only the TLR9 agonist ODN1826 also relied on type Ⅲ IFN signaling. This correlated with the finding that whereas all three types of agonists induced similar type Ⅰ IFN responses, the TLR9 agonist evoked higher type Ⅲ IFN responses compared to the agonists of the cytoplasmic sensors. Interestingly, the authors also found that strong induction of type Ⅲ IFN correlated with strong activation of the NF-κB pathway and a more modest activation of IRF-3, and that inhibition of the NF-κB pathway abrogated expression of type Ⅲ but not type Ⅰ IFN in vitro and in vivo, indicating that type Ⅰ and type Ⅲ IFN are induced by overlapping but non-identical signaling pathways (Iversen et al. 2010).
TLR3 is required to control HSV-1 particularly in neuronal tissues. Indeed, several studies demonstrated that HSV-1 encephalitis (HSE) is associated with TLR3 deficiency or lack of functional TLR3 signaling (Zhang et al. 2007). When human astrocytes were treated with agonists for TLR3, TLR7 or TLR9, the expression of IFN-λ was significantly induced by TLR3 activation (via poly I:C) and mediated an anti-HSV-1 innate immune response. Both IRF3 and IRF7 were found to be involved in poly I:Cinduced type Ⅲ IFN production (Li et al. 2012). In a murine in vivo model, a key role for TLR3 in IFN-λ production was re-confirmed, since deficiency of TLR3 resulted in abrogated IFN-λ production in sera upon poly I:C addition without a noticeable effect on the production of IFN-α (Lauterbach et al. 2010).
-
The RLR family consists of RIG-I and melanoma differentiation-associated gene 5 (MDA5) which recognize cytosolic dsRNA and, in the case of RIG-I, also 5'-triphosphated RNA. RIG-I and MDA5 interact with adaptor protein mitochondrial antiviral-signaling protein (MAVS), leading to the activation of NF-κB and IRF family members. HSV-1 produces dsRNA during replication, which was found to contribute to production of type Ⅰ IFN, type Ⅲ IFN and inflammatory cytokines in human monocyte-derived macrophages (Melchjorsen et al. 2006; Lauterbach et al. 2010). Indeed, whereas TLR2 and TLR9 were not involved in this process, knockdown of MAVS or MDA5 led to significantly lower levels of HSV-1-induced IFN-β and IFN-λ1 expression, while RIG-I knockdown had no effect (Melchjorsen et al. 2010).
-
The cGAS-STING pro-inflammatory pathway detects the presence of cytosolic DNA and can be triggered by herpesviruses like HSV-1 (e.g. (Sun et al. 2019). For VZV, it has been shown that infection of HaCaT keratinocyte cells triggers expression and secretion of IFN-λ, which is partly abrogated upon STING depletion via siRNA. Interestingly, whereas siRNA-mediated knockdown of RIG-I in MRC-5 cells did not affect VZV titers, siRNA against STING resulted in significantly increased virus titers (Kim et al. 2017).
Taken together, several pattern recognition receptors, including TLR3, TLR9, MDA5 and cGAS-STING play crucial roles in type Ⅲ IFN production during HSV/VZV infection, although the precise mechanisms are still unknown. It remains to be determined whether other PRRs that are able to sense herpesviruses, like IFI16 and DAI, may also contribute to IFN-λ production during herpesvirus infection.
-
The expression of type Ⅲ IFNs is driven by particular transcription factors that bind to the corresponding promoter regions, such as NF-κB and IRFs (Fig. 1). IRF3 and IRF7 have been implicated in the control of type Ⅰ IFN expression. IRF3/7 knockdown experiments showed that IRF3 knockdown only attenuates poly I:C-mediated IFNk1 expression, while IRF7 knockdown impaired both IFNk1 and IFN-λ2/3 expression in human primary astrocytes (Li et al. 2012). Later, Med23 was identified as a factor that significantly upregulates expression of the type Ⅲ interferon family at the mRNA and protein level by directly interacting with the transcription factor IRF7, and thereby contributes to the antiviral response against HSV-1 (Griffiths et al. 2013). A recent study suggests that ankyrin repeat domain 1 (ANKRD1) promotes optimal type Ⅰ/Ⅲ IFN production by working as a co-activator of IRF3 in viral recognition pathways in human primary antigen-presenting cells. Interestingly, ANKRD1 was significantly induced by HSV-1 infection, and silencing of ANKRD1 transcripts in primary dendritic cells enhanced HSV-1 viral load and decreased IFN-λ1 and IFN-β1 mRNA levels (Bin et al. 2018).
-
NF-κB acts as a key regulator of cellular processes in the immune response. In most unstimulated cells, NF-κB remains inactive in the cytoplasm through a direct interaction with inhibitors of NF-κB (IκB), while inducible degradation of IκB proteins by various stimuli, such as proinflammatory cytokines and viral and bacterial products, is a central mechanism regulating NF-κB transcriptional activity. NF-κB sites have been identified in the distal region of the IFN-λ1 promoter and were suggested to act independently of the proximal IFN regulatory factor 3/7 (IRF-3/7) binding sites, implicating different mechanisms of regulation between the IFN-β and IFN-λ1 genes (Thomson et al. 2009). In line with this, in the mouse system, knockdown of IRF3 has been reported to more potently impair type Ⅰ IFN expression compared to type Ⅲ IFN, whereas inhibition of the NF-κB pathway using the cell-permeable inhibitory NBD peptide abrogated expression of type Ⅲ but not type Ⅰ IFN both in vitro and in vivo, demonstrating that the NF-κB pathway plays a more dominant role in induction of IFN-λ than of IFN-α/β (Iversen et al. 2010).
-
A number of different cell types have been reported to produce type Ⅲ IFN in response to HSV infection to a more or lesser extent, including cells from the hemopoietic compartment (splenic cDCs, pDCs, T cells, B cells, and peritoneal macrophages) and the nonhemopoietic compartment (epithelial cells, fibroblasts, keratinocytes, lung carcinoma A549 and cervix epithelioid carcinoma HeLa) (Ank et al. 2006, 2008). Although epithelial cells are generally considered as major type Ⅲ IFN producers, cDCs and pDCs have been shown to also constitute important IFN-λ producers. Indeed, upon interaction with HSV, mice CD8α + cDCs and pDCs produced large amounts of type Ⅲ IFN (Ank et al. 2008; Lauterbach et al. 2010). When stimulating human PBMC with HSV-1, pDC were found to be the principal producers of IFN-λ1 (Megjugorac et al. 2009, 2010).
There are differences in amount and kinetics in type Ⅰ versus Ⅲ IFNs in different cell types/tissues during HSV infection. For example, purified human pDC were found to produce much more IFN-α than -k in response to HSV-1, but with similar kinetics (Yin et al. 2012). Following intravaginal infection of mice with HSV-2, IFN-λ was induced at low levels and with delayed kinetics compared to IFN-α. However, both IFN-α and IFN-λ were detectable at day 2 p.i., and IFN-λ showed a more prolonged kinetics of production with high levels still detectable at day 3 p.i (Iversen et al. 2010). These kinetics suggest that, at least during vaginal infection of HSV-2 in the mouse model, type Ⅲ IFN does not appear to serve as an earlier antiviral response compared to type Ⅰ IFN.
-
Using cell culture-based in vitro models, type Ⅲ IFNs have been shown to play a role in controlling HSV replication in different cell types, such as HepG2 cells (Ank et al. 2006) and cervical epithelial cells (End1/E6E7 cells) (Zhou et al. 2015; Li et al. 2017). In most studies, cultured cells were treated with IFN-λs and the impact of viral infection was assessed. Since the IFN-λ receptor (IFNLR1) is particularly expressed in epithelial cells, the anti-HSV-2 effect of type Ⅲ IFNs was investigated in vaginal epithelial cells. Interestingly, IFN-λ displayed a more potent antiviral effect than IFN-α in this cell type (Ank et al. 2008). In keratinocytes, type Ⅲ IFN was found to also display antiviral activity against HSV-1 (Zhang et al. 2011; Zhou et al. 2011). HSV-1, like most alphaherpesviruses, is a neurotropic virus that establishes latent infection in sensory neurons and may in rare cases cause herpes simplex encephalitis (HSE). Type Ⅲ IFN was found to suppress HSV-1 infection in human NT2-N neurons, CHP212 neuronal cells as well as primary human astrocytes and neurons (Li et al. 2011). The antiviral effect of type Ⅲ IFN against HSV-1 appears to be to some extent dependent on the pathogenicity of the HSV-1 strain. For example, IFNk1 induced a more potent antiviral activity in Vero cells against the highly pathogenic ANGpath strain compared to the moderately pathogenic KOS strain, which suggests that subtle differences in virus characteristics may play a role in the antiviral state induced by IFN-λ (Lopušná et al. 2014). IFN-λ1 and, to a lesser extent, IFN-λ2 also suppressed virus replication and viral gene expression and protein production in VZV-infected human dermal cells (Kim et al. 2017).
In vivo, the complexity of the role of type Ⅲ IFN in different tissues and between various immune cells during HSV infection has been explored using recombinant type Ⅲ IFN in IFNAR -/- and IFNLR -/- mouse model. Recombinant IFN-λ was found to provide protection against HSV-2 infection, even in IFNAR -/- mice, indicating that the anti-HSV-2 activity of IFN-λ was not dependent on a functional type Ⅰ IFN receptor system (Ank et al. 2006). However, based on the results obtained in the IFNLR1 -/- mouse model, endogenous type Ⅲ IFN does not seem to play an essential role in the HSV-2-activated host defense since IFNLR -/- mice did not show increased susceptibility to vaginal infection with HSV-2 compared to WT mice (Ank et al. 2008). More recently, miR-21 has been identified as a positive regulator of HSV1-induced production of both IFN-α and IFN-λ by pDC in mice (Liu et al. 2017). PTEN (phosphatase and tensin homolog) was found to be a major target for miR-21 in pDC and mice deficient in miR-21 showed impaired antiviral immune responses against HSV1 upon tail vein inoculation, illustrated by increased viral load in the brain and trigeminal ganglion, leading to increased body weight loss and reduced survival time of the mice (Liu et al. 2017).
With regard to betaherpesviruses, IFN-λ suppresses replication of HCMV and mouse cytomegalovirus (MCMV) in vitro in cells that express the IFN-λ receptor (Brand et al. 2005; Ding et al. 2014; Gimeno Brias et al. 2018). Infection of C57/BL6 mice with MCMV was found to trigger a nearly fourfold increase in IFN-λ3 mRNA levels in the colon of infected mice compared to those of noninfected mice at 48 h post inoculation (Brand et al. 2005). However, it is less clear to what extent IFN-λ contributes to antiviral immunity against beta-herpesviruses like MCMV in vivo. Indeed, although IFNLR1 -/- mice showed a defect in MCMV-induced development of IFNγ-expressing NK cells in the spleen, this did not impact the control of either acute or persistent MCMV replication (Gimeno Brias et al. 2018).
-
Our knowledge regarding the interaction of type Ⅲ IFN with herpesviruses is currently largely limited to studies on human alphaherpesviruses HSV-1 and HSV-2, with some studies on cytomegaloviruses. Few studies on other herpesviruses have been reported thus far.
In vitro, it has been shown that human plasmacytoid DCs induce strong IFN-α and IFN-λ1 responses upon contact with inactivated human herpesvirus type 6B (HHV- 6B), a betaherpesvirus, which were completely abolished in the presence of a TLR9-specific inhibitor, G-ODN (Nordström and Eriksson 2012).
Further, in vitro and in vivo antiviral activity has been reported for IFN-λ against murid herpesvirus-4 (MuHV-4), a gammaherpesvirus (Jacobs et al. 2019). In vitro, upon treatment with recombinant IFN-λ2, MuHV-4 infection was decreased by 65% in mouse LKR10 lung epithelial cells that overexpressed IFNLR1. In vivo, injection of a plasmid allowing long-lasting circulating mouse IFN-λ2 reduced upper respiratory tract infection, but not lung infection, upon intranasal MuHV-4 inoculation. In the same study, circulating mouse IFN-λ2 failed to block genital reactivation of MuHV-4 although it did appear to delay reactivation (Jacobs et al. 2019).
Virtually all studies on the interaction of herpesviruses with type Ⅲ IFN have been performed in man and mouse. We are aware of only one study in another species. In vitro, treatment of duck embryo fibroblasts with recombinant goose IFN-λ was found to provide a certain degree of protection against infection with the alphaherpesvirus DPV in a dose-dependent manner (Chen et al. 2017).
Toll-like Receptors (TLRs)
Retinoic Acid-Inducible Gene I (RIG-I) Like Receptors (RLRs)
Cyclic GMP-AMP Synthase (cGAS)-Stimulator of Interferon Genes (STING)
Transcription Factors that are Involved in Herpesvirus-Induced Type Ⅲ IFN Production
IRF3/7
NF-κB
Cell Types that Produce Type Ⅲ IFN Upon Herpesvirus Infection
Antiviral Effects of Type Ⅲ IFN Against Herpesvirus Infection in vitro and in vivo
Type Ⅲ IFN and Other Herpesviruses
-
Based on the available data thus far, it is clear that herpesvirus infection, at least HSV-1 and HSV-2 infection, is associated with substantial type Ⅲ IFN responses that are triggered by different cell types, including epithelial cells, pDC and cDC, and that these responses contribute to the (local) antiviral defense against these pathogens. Although mechanisms of type Ⅰ and Ⅲ IFN production during HSV infection substantially overlap, they also show interesting differences, with type Ⅲ IFN responses relying more on NF-κB signaling compared to the type Ⅰ IFN response.
Despite their predominantly local innate effects, there are indications that type Ⅲ IFNs may be of relevance in prophylactic strategies against HSV. The medical, economic and social consequences of HSV-1 and HSV-2 infections, in combination with their widespread prevalence – with estimates that roughly two thirds of the global population is infected with HSV-1 and ~ 11% of the world population is infected with HSV-2 (Looker et al. 2015)—has led to several but thus far largely disappointing attempts to design vaccines against these pathogens. To date, more than 10 HSV preventive vaccines have been developed and evaluated in human clinical trials (Johnston et al. 2016). Quite recently, IFN-λ has been evaluated as an adjuvant for a DNA vaccine against HSV-2, where treatment with IFN-λ3 was found to enhance HSV-induced expression of IFN-γ, a cytokine that is central in a successful adaptive response against these viruses. Indeed, intramuscular injection of the recombinant DNA vaccine gD2DUL25/IL28B encoding the HSV-2 glycoprotein gD and capsid vertex protein UL25 in combination with IFNk3 was found to enhance HSV-specific IgG production, IFN-γ synthesis by T cells and protection against an intravaginal challenge infection compared to vaccine preparations that lacked IFN-λ3 expression in a mouse model of HSV-2 infection (Zhou et al. 2017). On the other hand, when considering use/induction of IFN-λ as an antiviral strategy, potential harmful side-effects should not be overlooked. Indeed, despite the limited cell types that produce and respond to IFN-λ, in comparison with the much broader spectrum of producing and responsive cell types for type Ⅰ IFNs, local IFN-λ-induced inflammation and tissue damage cannot be excluded. For example, herpesviruses occur in periodontal pockets of patients with chronic or aggressive periodontitis and higher levels of IFN-λ transcripts have been found in gingival tissues of patients with chronic or aggressive periodontitis compared to healthy controls, suggesting a role for (virus-induced) IFN-λ in periodontitis (Bilichodmath et al. 2018). On the other hand, another study found that IFN-λ1 protein levels were lower in periodontitis patients that were positive for HSV1, HSV2, HCMV and/or EBV compared to virusnegative periodontitis patients, suggesting that IFN-λ1 may have effective antiviral and therapeutic value against herpesviruses in the pathogenesis of chronic periodontitis (Jayanthi et al. 2017). Clearly, it will be important to carefully discriminate beneficial, anti-viral and disease-resolving effects of IFN-λ from potentially harmful chronic inflammatory responses in the context of periodontitis.
Seen the efficiency of host invasion and transmission associated with herpesviruses, it comes as no surprise that herpesviruses display several strategies to thwart several key elements of the innate and adaptive immune system (De Pelsmaeker et al. 2018; Pontejo et al. 2018; Berry et al. 2020; Jones 2019; Liu et al. 2019; Tognarelli et al. 2019; Gonzalez-Perez et al. 2020). Indeed, research on the importance of type Ⅰ IFN during herpesvirus infections has gone hand in hand with the discovery of mechanisms that these viruses use to suppress production, signaling and/or antiviral effects of type Ⅰ IFN (García-Sastre 2017; Danastas et al. 2020). As far as we know, there are currently no reports specifically addressing herpesvirus evasion of the type Ⅲ IFN system. Quite contrary, whereas murine gammaherpesvirus infection of mouse mammary epithelial cells leads to rapid degradation of the type Ⅰ IFN receptor, no degradation of the type Ⅲ IFN receptor was observed (Lopušná et al. 2016). Whereas the type Ⅰ and type Ⅲ IFN receptors are very different, the receptortriggered type Ⅰ and Ⅲ IFN signaling mechanisms and antiviral effects are very similar, making it reasonable to assume that at least some of the reported herpesvirus evasion mechanisms of the type Ⅰ IFN system also hold true for type Ⅲ IFN, as has been reported for the KSHV vIRF-2 homologue of IRF, which inhibits interferon signaling by both type Ⅰ and type Ⅲ IFNs (Fuld et al. 2006). Additional herpesvirus evasion mechanisms that specifically interfere with the type Ⅲ IFN system likely await discovery.
A hallmark of herpesviruses is their ability to establish lifelong, latent infections in particular cells and tissues of their natural host. No infectious virus is produced during latency, and specific stimuli and/or changes in the immune defence of the host (e.g. immunosuppression) can trigger reactivation of latent virus, which may lead to infectious virus production and virus spread and transmission. Betaand gammaherpesviruses establish latency in hematopoietic cells, whereas most alphaherpesviruses target peripheral neurons. Several factors control the switch between latent and productive herpesvirus infection (Koyuncu et al. 2018). One of the most important and best characterized factors that contribute to latency establishment and suppression of reactivation, particularly in the case of alphaherpesviruses, are interferons (Enquist and Leib 2017; Koyuncu et al. 2018). However, despite several studies showing that type Ⅰ and type Ⅱ IFN promote alphaherpesvirus latency and/or prevent reactivation from latency (e.g. Liu et al. 2019; De Regge et al. 2010; Song et al. 2016; Enquist and Leib 2017; Linderman et al. 2017; Koyuncu et al. 2018), evidence that type Ⅰ IFN also contributes to latency of the betaherpesvirus MCMV (Daǧ et al. 2014; Murray et al. 2018) and that type Ⅱ IFN suppresses murine gammaherpesvirus 68 reactivation from latency (Steed et al. 2006), no studies have specifically addressed the possible role of type Ⅲ IFN in this context. Some reports have suggested low level expression of IFN-λ and the type Ⅲ IFN receptor in neurons (of the CNS) (Sorgeloos et al. 2013), but it is unclear at this stage whether type Ⅲ IFN may or may not contribute to (alpha-) herpesvirus latency.
Whereas our knowledge on the interaction between HSV and type Ⅲ IFN in both mouse and man is increasing but still incomplete, there is a stunning lack of knowledge regarding this interaction for other herpesviruses. More studies based on other herpesviruses (alphaherpesviruses from other species as well as herpesviruses from other subfamilies) are urgently needed to evaluate whether the accumulating evidence regarding the importance of type Ⅲ IFN in HSV pathogenesis, and the potential of type Ⅲ IFNs as adjuvant in (DNA) vaccines may also hold true for other herpesviruses and in other species.
-
YY is supported by a PhD scholarship grant of the China Scholarship Council (CSC). Research of YY and HWF is supported by grants from the Special Research Fund of Ghent University (CSC-cofunding grant and Concerted Research Action GOA013-17).
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: