-
Hepatitis B is an infectious disease of the liver caused by Hepatitis B virus(HBV). Globally,about two billion people have been infected by HBV and approximately 350 million people are chronic carriers of HBV (23, 2, 19, 5). These carriers are at high risk for development of HBV-associated diseases,including liver cirrhosis,liver failure and hepatocellular carcinoma(HCC) and more than one million carriers die from these diseases each year (23, 2). In China,the prevalence of HBV infection is very severe,with an estimated 120 million chronically infected carriers,up to 12 million suffering from chronic hepatitis B (5).
Interferon and lamivudine are the mainly acceptable antiviral drugs for inhibition of HBV replication (14, 8). Both of drugs can induce virological and biochemical remission in the treatment of chromic hepatitis B,but their clinical chromic hepatitis B,but their clinical benefit is somewhat limited due to viral mutation,cost,frequent adverse effects (7) and the likelihood of re-lapse after the termination of treatment. Moreover,only about 20% of patients benefit from combination therapy with interferon and lamivudine (13, 9). So,the need to identify effective anti-HBV agents is still urgently required. The herbal plant Rheum palmatum L. is a traditional Chinese medicine which is widely distributed in mainland China and which has a long history to treating gastroenteritic and viral diseases (4). Rheum palmatum L. contains several anthraquinone derivatives,which have showed activities against some viruses including vesicular stomatitis virus,herpes simplex virus types 1 and 2,parainfluenza,vaccinia virus,human cytomegalovirus and poliovirus (1, 3, 20, 22). It was also reported that Rheum palmatum L. volatile oil could inhibit HBV antigens(HBsAg and HBeAg) expression (24). Therefore,it is supposed that Rheum palmatum L. might possess active agents which may inhibit many viruses including HBV. In this study,we evaluated the cytotoxicity and the inhibitory activities of Rheum palmatum L. ethanol extract(RPE) against HBV-DNA replication and HBV antigens expression in a stable HBV-producing cell line HepAD38,and compared these activities with the commercial anthraquinone derivatives emodin and rhein,which are derived from rheum.
HTML
-
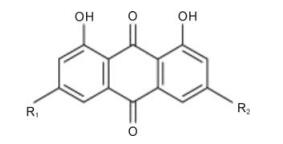
The herbal plant Rheum palmatum L. was purchased from a herbal materials supply house and verified. One kilogram of dried,ground rhizome was macerated in 1 000 mL 95% ethanol. After incubation overnight,the sample was subjected to ultrasonication for 30 minutes,followed by centrifugation. The residue was extracted twice,then the supernatant solutions were mixed,filtered,concentrated and lyophilized to give a dark brown powder. The dried extract was re-resolved in Dulbecco's modified Eagle's medium(DMEM,Gibco BRL) with 0.2% dimethyl sulfoxide(DMSO,Sigma,St. Louis,MO) to the appropriate concentrations.Emodin[1,3,8-trihydroxy-6-methylanthraquinone,C15H10O5) and rhein (1,8-dihydroxy-9,10-anthraquinone-3-carboxylic acid,C15H8O6) (Fig. 1) were purchased from Sigma Chemical Company and dissolved in DMEM with 0.2% DMSO to the appropriate concentrations. The resulting solutions were used in the following analysis.
-
The HepAD38 is a human hepatocellular carcinoma derivative cell line which can reproduce hepatitis B viruses under control of tetracycline (11). HepAD38 cells were maintained in DMEM supplemented with 10% FBS,1% penicillin/streptomycin,1% glutamine,100μg /mL kanamycin,400μg /mL G418 and 0.3μg /mL tetracycline at 37℃ in an atmosphere of 5% CO2and 100% humidity. When the assay was started,cells were washed with pre-warmed phosphate-buffered saline(PBS) and fed with medium without tetracycline. HBV replication in HepAD38 cells was induced by withdrawal of tetracycline from the culture medium.
-
Prior to the investigation of anti-HBV effects,the cell growth inhibiting effects of the Rheum palmatum L. ethanol extract(RPE) and the anthraquinone derivatives were determined with the MTT assay (15). Briefly,the cells were plated in 96-well tissue culture plates at a density of 1×104 cells/well in DMEM culture medium and allowed to incubate for 48 hours. RPE and anthraquinone solutions were added to various final concentrations in triplicates and DMEM with 0.2% DMSO was used as control. After incubation,20μL 3-(4,5 -dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added into each well and further incubated at 37℃ for another 4 hours. Then,150μL DMSO was added and the solution was shaken for 10 minutes to dissolve the MTT formazan crystal. The absorbance was measu-red by a microplate reader(PE Biosystems,Foster City,CA) at 570 nm with a 650 nm reference.
-
HepAD38 cells were inoculated at a density of 1×105 cells/well in 24-well tissue culture plates. The RPE and the anthraquinone solutions were added to the mediums after 2 days incubation. Then,the cells were grown in the presence of these drugs for 8 days with the medium changed every two days. The culture mediums were collected after 8 days treatment and separated by centrifugation at 10 000×g at 4℃ for 5 minutes. An aliquot of the culture medium was used for estimation of HBsAg and HBeAg. The remaining medium was processed to obtain HBV virions by using the polyethylene glycol (PEG) precipitation method (6). The viral DNA recovered from the secreted HBV particles was quantified by real-time PCR using a commer-cial real-time PCR HBV diagnostic kit (PG Biotech,Shenzhen,China) and the amplification was perfor-med according to the manufacturer's instruction.
-
After treatment of RPE and anthraquinone solutions,culture medium was collected and clarified as described above. The levels of HBsAg and HBeAg were determined by the enzyme linked immunosorbent assay(ELISA) kits and performed according to the protocol provided with the kits(Abbott Murex,Wiesbaden,Germany).
-
The results were calculated as the percentage of controls and expressed as the mean ±SE of independent experiments. Data were analyzed by the Paired-samples t test using the Spss 12.0 software program(Spss Inc,Chicago,IL). Statistical significance was defined as P < 0.05 compared with control values.
1.1. Preparation of herbal plant extract and anthraquinone solutions
1.2. Cells culture
1.3. Cell viability assay
1.4. Quantification of extracellular HBV-DNA
1.5. Measurement of HBsAg and HBeAg
1.6. Statistical analysis
-
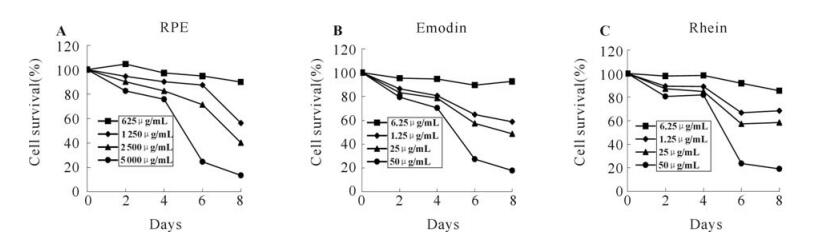
The cell growth inhibitory effects of the RPE(Rheum palmatum L. ethanol extract),emodin and rhein were measured to determine the anti-HBV treatment concentrations in the cell culture system. HepAD38 cells were incubated with various concentrations of emodin,rhein(0,6.25,12. 5,25 and 50μg/mL) and RPE (0,625,1 250,2 500 and 5 000 μg/mL) for 2,4,6 and 8 days and the cells viability was determined by MTT assay. The dose-dependent inhibit effects of these drugs on the HepAD38 cells were shown in Fig. 2. Compared with the 50% cytotoxic concentration(CC50) values of emodin and rhein(22.78±1.9 and 28.94±5.1μg/mL,respec-tively),RPE showed a weak cytotoxic effect on HepAD38 cells(CC50=1 640±114μg/mL) and the CC50 value was about 80 fold higher than emodin and rhein.
-
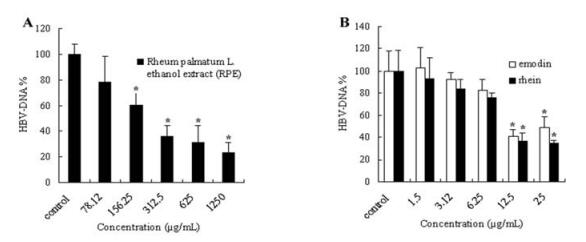
Based on the results obtained from the MTT assay,six appropriate concentrations of these drugs were adopted for the anti-HBV assay. HepAD38 cells were inoculated in 24-well plates and treated with emodin,rhein(0,1.5,3.12,6.25,12.5 and 25μg /mL) and RPE (0,78.12,156.25,312.5,625 and 1 250μg /mL). The extra-cellular viral DNA was quantified by real-time PCR based on the TaqMan technology after 8 days treatment. As shown in Fig. 3.A,RPE could significantly inhibit the replication of HBV-DNA in the HepAD38 cell line and produce a dose-dependent inhibition at the concentrations ranging from 78.12 to 1,250μg /mL. The concentration of 50 % inhibition(IC50) of RPE was calculated to be 209.63±14.26 g/mL. Emodin and rhein also showed their inhibitory activity against replication of HBV-DNA,although not as strong as RPE. At high concentration,Rhein showed higher activity than emodin. The IC50 values of emodin and rhein were calculated to be 10.48±1.68 and 9.89±2.35μg /mL,respectively (Fig. 3.B).
-
The expression levels of HBsAg and HBeAg in the mediums were also measured after treated by the different concentrations of RPE,emodin and rhein as described above. Compared to the control groups,RPE showed a marked effect for reducing HBsAg levels at the concentration of 312.5μg /mL(P < 0.05) (Fig. 4.A). The IC50 of RPE on HBsAg was 252.53±10.23 μg/mL. Rhein had a slight effect on HBsAg at concentrations greater than 12.5μg /mL (Fig. 4.C). Emodin showed no significant inhibitory effect on HBsAg expression even at the highest concentration of 25μg /mL (Fig. 4.B). Moreover,all three samples showed weak inhibitory activities of HBeAg expression (Fig. 4).
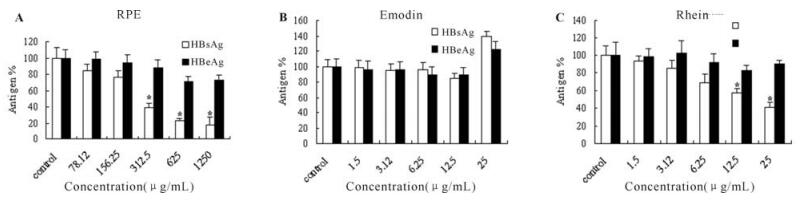
Figure 4. Effect of RPE (A),emodin (B) and rhein (C) on the levels of HBsAg and HBeAg in the culture medium. The results from three independent experiments were calculated by percentage of control and expressed as means ± SE. (*) indicates significant different between test and control (DMEM with 0.2% DMSO) at p < 0.05.
2.1. Effects of RPE,emodin and rhein on cell viability
2.2. Inhibitory effects of RPE,emodin and rhein on extracellular HBV-DNA
2.3. Effect of RPE,emodin and rhein on HbsAg and HBeAg expression
-
Since the non-human host range of HBV is restricted to a few animals,such as chimpanzee,several cell lines were developed to identify potential therapeutics against HBV replication. The HepG2 2.2.15 cell line,which constitutively produces HBV Dane particles,is the most well-known. But because of the low level of virus replication in this cell line,the assay usually requires relatively large amounts of cells and consumes more time before an anti-HBV effect can be detected (10). The HepAD38 is a stably transfected cell line that contains a cDNA copy of the HBV pgRNA under the control of a tetracycline-responsive promoter. These cells support the replica-tion of HBV in the absence of tetracycline and they could produce approximately 11-fold more viral DNA than HepG2 2.2.15 cells after 7 days incubation [11). Moreover,the HepAD38 cell line provides a safer cell-based assay because of its ability to strictly regulate the commencement of HBV replication.
The routine approach for screening agents to treat HBV infection is based on consideration of their specific activities against HBV-DNA replication and antigens expression (10, 12). Compared with the control groups,Rheum palmatum L. ethanol extract (RPE) could inhibit about 80% HBV-DNA replication and HBsAg expression at the concentration of 1 250μg /mL. Meanwhile,no significant cytotoxicity was observed at 50% HBV-DNA inhibitory concentra-tion(IC50= 209.63±14.26μg /mL),which indicated that the inhibitory activity of HBV-DNA replication and HBsAg expression was not due to the cytotoxicity. The selectivity index(SI) is an important parameter as it considers both antiviral activity and eventual toxicity of the test compound. The SI value is defined as the ratio of CC50 to IC50,so,the higher the value of SI,the higher the effect against the virus and the lower the toxicity to the host cells. The SI of RPE was 7.82,noticeably higher than the SI values of emodin and rhein(2.17,2.93,respectively). Since RPE possesses high anti-HBV effect and weak cytotoxicity,this suggests its considerable potential for the treatment of HBV infection and the extract might be fit for a long-term treatment.
In most studies of HBV inhibitory agents,cytotoxi-city assays were performed 2 or 3 days after agents' treatment,while the results of anti-HBV assays were obtained after 8-10 days treatment. The agents mightshow weaker cytotoxicity because of shorter treatment time. In our study,cytotoxicity was was determined over a period of 8 days equivalent to the period for the anti-HBV assay. Therefore,the results could more accurately represent the relation-ship between inhibitory activities and cytotoxicity of RPE,emodin and rhein. Cytotoxicity might also help to explain why the highest percentage of viral antigens was detected at the concentration of 25 μg /mL emodin in Fig. 4B. This concentration is higher than the emodin CC50 on HepAD38 cells,so it was supposed that significant cells lysis caused by emodin would release more of the viral antigens into the culture medium and affect the ELISA result.
In Rheum palmatum L. ethanol extract(RPE),the bulk of the phytoconstituents are anthraquinones,consisting of free anthraquinones such as emodin,rhein,chrysophanol,physcion,aloe-emodin,and combined anthraquinones including anthraquinone glycosides and so on (16, 17). The anthraquinones were supposed to be antiviral agents,and they could inhibit different viruses in different ways (1, 3, 18, 20). For example,the polyphenolic and polysulfonate substituted anthraquinones could inhibit the activity of human immunodeficiency virus 1 transcriptase (18) and aloe-emodin could inhibit viruses by disrupting their envelopes. In our study,the RPE showed more activates of HBV inhibition and lower cytotoxicity than emodin and rhein,which maybe suggests that there may be other active compounds in the RPE. Moreover,the ingredients of RPE may be different depending on the methods of extraction. Usually,for extraction of the traditional herbal medicine,a long decoction time is employed. But this method will affect the pharmacodynamics of Rheum palmatum L.,due to the hydrolysis of combined anthraquinones,the decomposition of free anthraquinones and the inter-transition among anthraquinones (21). In this study,the ultrasonication extraction method was used to avoid lengthy periods of boiling. As a result,more combined anthraquinones could remain in the RPE. According to the data,the RPE could inhibit HBV more efficiently than emodin and rhein,indicating the functional agents in the RPE might be combined anthraquinones. As the actual mechanism of anthra-quinones inhibition of viruses is still unclear,it is worthwhile to isolate the active anthraquinones from the Rheum palmatum L. ethanol extract(RPE) in a future study and to identify new agents against HBV infection.













 DownLoad:
DownLoad: