-
Since it was identified in Taiwan in the early 1990's,White spot syndrome virus (WSSV) has spread throughout the world and has become the main pathogen in shrimp cultures. It has a wide host range and can infect most aquatic crustacean species.The infected shrimps reach an accumulative mortality of 100% within 3 to 10 days (1, 3, 4, 8, 21). WSSV has a genome size of 305kb,the biggest genome of animal viruses (12, 19). According to the morphology and genome structures,WSSV has been assigned to a new virus family Nimaviridae by ICTV (9). The study of virucation and assembly are hampered because of lacking permissive shrimp cell lines. At least 39 structural proteins have been identified with proteomics methods,of these proteins,7 were reported to be involved in virus infection (2, 6, 5, 10, 13, 17). VP28 is the main envelope protein of WSSV,and an target for developing diagnostic methods (11, 20). Previous reports have shown that anti-VP28 polyclonal antibodies could neutralize the virus and delay the death of infected shrimp (13). Expressed VP28 and its subunit in prokaryotic cells can protect shrimp from WSSV (16) ,suggesting that VP28 has an important role during the virus entry. In this paper,six anti-VP28 monoclonal antibodies have been screened by ELISA with VP28 expressed in procaryotic cells and their characteristics have been identified.
HTML
-
The crayfish Procambarus clarkii was used in viral proliferation and neutralization assays. Crayfish were purchased from a Wuhan market (Hubei Province,China). They were reared for at least 3 days before the experiment. BALB/c mouse and Kunming mouse were purchased from the Center for the Disease Control in Hubei Province.The SP2/0 cell line was from the Wuhan Institute of Biological Products.
-
Virus proliferation and purification were performed according to the protocol described by reference (18).
-
Six-week BALB/c mice were immunized with purified virus. 30µg virus was injected into the spleen of each mouse. After 3 weeks,each mouse was injected subcutaneously with 50µg virus mixed with Freund's adjuvant. This was repeated once more after another 3 weeks. Ten days after third immunization,one mouse was sacrificed for antibody titer assay. The serum was kept as positive control. One immunization was added 3 days before the cell fusion.
-
The cell feeder was prepared one day before fusion. Cell fusion was performed with spleen cell and SP2/0 in a ratio of 4:1 by using PEG 1 500. Hybridomas were cultured in RPMI1640 medium with a complement of 20% FCS and HAT(Invitrogen). The positive clones were screened by ELISA with the VP28 expressed in E.coli (5µg/mL).
-
A volume of 0.5ml pristane was injected into the BALB/c mouse paunch. After 7 days,105 hybridomas were injected into the BALB/c mouse paunch to produce ascites. Ten days later,the mouse was sacrificed and the ascites were collect.
-
The immunoglobulin isotype of the monoclonal antibodies(mAb) was determined using the mouse monoclonal antibody isotyping kit(HyCult biotechnology b.v.) according to the manufacturer's instructions. The antibody titer was tested by ELISA with diluted purified virus.
-
Purified virus and expressed VP28 protein were transferred to PVDF membrane after 12% SDS-PAGE. Western blot was performed with 6 mAbs supernatant as the first antibody and goat anti mouse HRP-IgG as the second antibody. Polyclonal antibody against WSSV was used as positive control and non-specific supernatant as negative control.
-
One microlitre of purified virus was added to a 1×1 cm PVDF membrane. The ascites were diluted in 1:50 and added as the first antibody to perform a dot blot. The polyclonal antibody was used as positive control and non-specific ascites as negative control.
-
The in vivo neutralization assay using 6 mAbs was performed as described by Huang et al (2). A volume of 100µL virus (104 particles per microliter) was mixed with 100µL ascites diluted in TNE buffer (0.5 mol/L Tris-HCL,0.1 mol/L NaCL,0.01mol/L EDTA,pH 7.4) and incubated at 37℃ for 2 h. The mix of virus and non-specific ascites were injected as positive control. The mix of non-specific ascites and TNE were injected as negative control. Ten crayfish were used for each group and three replicates were performed. Crayfish mortality was monitored every day.
-
The mAb 7B4 was purified using the antibody puri-fication kit (Oncogene) according to the manufac-turer's instructions. The purified antibody was labeled with 15 nm colloidal gold according to the manufac-turer's protocol (14).
-
Purified viral particles and nucleocapsids were absorbed to Formvar-supported and carbon coated nickel grids for 10 minutes and then blocked with 4% BSA (in PBS pH7.4) for 30 minutes. The grid was incubated with 4-fold diluted mAb 7B4 (in TBS,pH8.2,) conjugated with colloidal gold for 60 minutes,and then washed three times with TBS (Tris-Base 20 mmol/L,NaCl 137 mmol/L,pH8.2,with 0.1% BSA) for 5 minutes. After extensively washing several times,the grid was stained with 2% phosphotungstic acid (pH 7.2) for 3 min and then observed with electron microscopy. The colloidal gold labeled BALB/c mouse IgG was used as negative control.
1.1. Materials
1.2. Virus proliferation and purification
1.3. Immunization
1.4. Cell fusion and positive clone screening
1.5. Ascites production
1.6. Isotype determination and titer test
1.7. Western blot
1.8. Dot blot
1.9. Neutralization assay
1.10. Purification of mAb and labeling with colloi-dal gold
1.11. Localization of VP28 on virus
-
We obtained 370 hybridomas after 2 fusions and succeeded in establishing 6 stable clones designated 5B7,3A10,1E7,7E12,7B4 and 6F6. All the 6 clones were characterized and used for neutralization assays. The titers of 6 clones determined by ELISA were between 104~106. The results are shown in Table 1.

Table 1. Moooclonal antibody isotype and titer
-
Purified virus and expressed vp28 protein were transferred to PVDF membrane after 12% SDS-PAGE. Western blot was performed with 6 mAbs as first antibody,but no positive signal appeared. This suggested that all these monoclonal antibodies were against the conformational structure of VP28. Denatured VP28 protein cannot be recognized by the 6 mAbs.
-
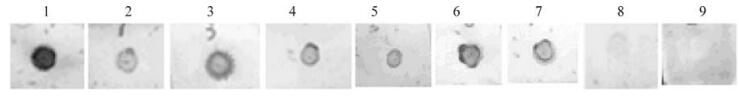
All 6 mAbs reacted strongly with virus. But no reaction was detected to negative control. This suggested that all these monoclonal antibodies were specifically against virus protein (Fig. 1.).
-
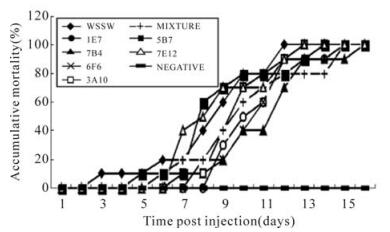
The results of the neutralization assay are shown in Fig. 2. No crayfish died in the negative control,i.e. those injected with non-specific ascites and TNE. The group injected with virus and non-specific ascites showed 100% mortality at day 12 post-infection(p.i.). The neutralization assay with mAbs 3A10,1E7,7B4,and 6F6 showed a delayed onset of mortality compared with positive controls. However,the mAbs of 5B7 and 7E12 didn't show any inhibition effect on virus in the assay.
-
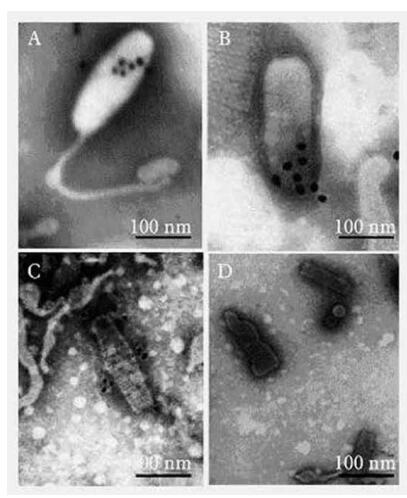
The results are shown in Fig 3. The mAb 7B4 labeled with colloidal gold particles can be localized to VP28 on the intact virus envelope and the collapsed envelope protein from the virus (Fig. 3.A,B,C). The gold-labeled BALB/c mouse IgG did not localize on the viral particles (Fig. 3.D).
2.1. Hybridomas screening,isotype and titer deter-mination of mAb
2.2. Western blot
2.3. Dot blot
2.4. Neutralization assay
2.5. Localization of VP28 on virus
-
Since its detection,the prevention and control of WSSV has been an important issue in aquaculture. Unfortuna-tely,no efficient methods have been found to control this viral disease. Thus,a prophylactic strategy is the only available option to minimize the economic losses of this disease. The six mAbs obtained in this study could be used to develop immunological diagnosis methods of WSSV infection based on techniques such as ELISA.
Envelope proteins usually play key role in infection,such as recognizing and attaching to the receptor,and fusing with cell membrane during assembly,etc. Pre-vious studies showed that VP28 is involved in virus infection. VP28 expressed in vitro can protect crayfish from WSSV infection and induce a significant anti-virus response in crayfish (13, 15, 16). Thus,further study of VP28 function is helpful to understand virus infection and to develop WSSV prevention and control methods. In 2002,Liu et al expressed recombinant VP28 protein(r-28) in Escherichia coli and used it as an antigen to obtain 3 monoclonal antibodies,and an antigen-capture ELISA(Ac-ELISA) was developed by virtue of these mAbs. The Ac-ELISA can differentiate WSSV-infected shrimp from uninfected shrimps and has the same sensitivity as the PCR assay (7). In our study,we used viral particles as antigen and screened mAbs against VP28. Western blot and dot blot demonstrated that all these monoclonal antibodies were against the conformational epitope of VP28. The in vivo neutralization assay using 6 mAbs in crayfish showed that mAbs 3A10,1E7,7B4,and 6F6 could significantly delay initial mortality compared with positive controls. It implies that the epitopes of these 4 mAbs may play an impor-tant role in the virus infection and can be used for further study of VP28 function,virus entry and development of anti-virus methods.













 DownLoad:
DownLoad: