-
Classical swine fever (CSF) is a highly contagious and often fatal disease of swine. It is caused by classical swine fever virus (CSFV), one of the members of the genus Pestivirus of the Flaviviridae family (1). An epidemic often causes huge economic losses, so CSF is one of 16 OIE list A diseases and under strict surveillance (7). The genome of CSFV, a positive-stranded RNA genome, comprises a single long open reading frame (ORF) coding for a poly-protein encompassing all the viral proteins. The structural proteins comprise the nucleocapsid protein C and three envelope glycoproteins, Erns, E1, and E2. Erns and E2 are located at the surface of infected cells (12), induce virus-neutralizing antibodies and mount protective immunity in the natural host (2, 5, 11).
Studies have showed that CSFV envelope gly-coprotein E2 containing one structural antigenic unit protects pigs from lethal CSFV challenge (9). With a panel of 13 MAbs, four antigenic domains, A, B, C and D, have been identified on the E2 gene of CSFV strain Brescia. Domains A, B and C contain epitopes for neutralizing MAbs (13). These antigenic domains have been mapped to the N-terminal half of E2 and are located on two independent structural units in a proposed model of the antigenic structure of E2 (10). Until now, attenuated live vaccines have been the most effective weapons to defend against pathogen infection. However, Genghini et al reported obvious pig chromosome aberrations after vaccination with live vaccine, which serves as a reminder of the potential danger of live vaccines. So it is necessary to find an alterative safer vaccine against CSFV.
Retroviral vectors are believed to be advantageous for single gene transfer, which may allow for the simultaneous achievement of more efficientgene delivery and longer-term transgene expression. The ability of retrovirus vectors delivering a particular gene to target cells has been applied commonly in both experimental and clinical settings. In this report, we have cloned and expressed four major antigen domains of E2 gene of CSFV in eukaryotic cells, and analyzed the immunological activity in rabbits. The antigenicity and immunogenicity of the construct was monitored in rabbits in a first step to evaluate the possibility of developing a subunit vaccine.
HTML
-
GP2-293 cells; PK15 cells; pBABE puro vector and pVSV-G plasmid were preserved at the Virology Department of Lanzhou Veterinary Research Institute, Gansu province in Lanzhou City in P.R. China. 5-diphenyltetre zolium bromide (MTT); 3-(4, 5-Dimethylthiazoyl2-yl)-2, Dimethylsulfoxide (DMSO) and phytohemagglutinin (PHA) were purchased from Sigma Chemical Co., USA; the CSFV Antigen and Antibody Test Kit was purchased from IDEXX Laboratories, Ltd., Sweden.
-
The rabbit experiment included three sequential periods: the acclimatization period (1 week), the im-munization period (4 weeks) and the post-inoculation period (4 weeks). Thirty healthy rabbits weighing between 400-500g were randomly divided into three groups (ten rabbits per group). Each group was raised separately. During the acclimatization period (1 week), environmental temperatures and individual body temperatures were monitored once a day. The normal rectal temperature was at about 39.0±0.5℃, and a temperature exceeding 41℃ was considered as fever.
-
GP2-293 cells and PK-15 cells were cultured in DMEM supplement with 10% fetal calf serum. CSFV Shimen strain virus (Sequence number in Genbank: AF092448) and C-strain virus were preserved at the Virology department of Lanzhou Veterinary Research Institute, Gansu, China.
-
E2 gene was amplified by PCR using two oligonucleotides from the CSFV Shimen strain. The 5′ primer (E2-f: 5′-TGGAATTCCACCATGCGTCTAGC CTGCA-3′) contained a sequence for an EcoR I restriction site, while the 3′ primer (E2-r: 5′-ATGGATCC ATAGATCTTCATTTTCCACTGTGG-3′) contained a BamH I restriction site. A KOZAK consensus sequence (GCCGCCACC) was designed to be in-cluded upstream of the initiation codon of E2-f for efficient translation initiation in the vector (6). The constructed recombinant plasmid pBABE puro-E2, was sequenced to make sure that no mutations were generated during PCR amplification and cloning. Plasmid DNA for transfection was isolated by S.N.A.P. Miniprep Kit (Invitrogen) and concentration was determined by spectrophotometry at A260 and A280.
-
The GP2-293 cells were plated at 1:10 (approx. 9×105 cells per 30 mm plate) for 24 h before trans-fection. Then co-transfection of the mixture (recombi-nant plasmid pBABE puro-E2 and pVSV-G) was performed in PK15 cells with liposome according to the manufacturer's protocol (Invitrogen). The viruses were collected 72 h after transfection, and the media was replaced with 5 mL of fresh DMEM (complete). Viruses were collected about every 5 h for 2 days and frozen in sterile tubes at –70℃.
-
PK15 cells to be infected were split within 24 h before infection and grown to 20% confluency for infection so that they did not overgrow the plate before being cultured in selective media. Medium was replaced with 2 mL of artificial viral supernatant plus enough growth media to cover the cells for a 30 mm plate, polybrene was added to a concentration of 8μg/ mL. The media was changed once a day for the first 2 or 3 days after infection. A positive PK15 cell colony was produced during the following 2 to 4 weeks and named PK15-E2.
-
The positive cells were analyzed for expression of CSFV E2 proteins by the indirect immunofluo-rescence test (IFAT) and sandwich-ELISA. For IFAT, monolayer of PK15-E2 cells were cultured on cover slips, fixed in 100% cold acetone (−20℃ for 30 min), and then the sample was stained with fluorescence conjugated FITC CSFV mAbs for 30 min at 37℃ in a humid box. For sandwich-ELISA, the PK15-E2 cells were washed 48 h after culture. The particular procedure followed the IDXX CSFV antigen kit handbook. For detecting the genetic stability of E2 gene, the genomic DNA of PK15 cells was distilled by U-gene DNA distill kits, and the CSFV E2 gene was identified by PCR using P1-f and P1-r as primers. The genetic stability was also monitored by PCR using DNA from the 1st, 10th, 20th and 30th passages.
-
Positive cell lysates were electrophoresed in 10% SDS–polyacrylamide gels and transferred to a nitrocellulose membrane (Bio-Rad), the membrane was incubated for 1 h in blocking solution (5% non-fat dry milk, 0.1% Tween-20 in phosphate buffered saline, PBS), with 100-fold dilution of swine immune serum, and with 800-fold dilution of anti-swine IgG rabbit antibodies conjugated to horseradish peroxidase (Sigma, USA). Finally, the membrane was soaked in TMB solution for color development.
-
During the immunization period, these conjugants were mixed with complete Freund's adjuvant (CFA) for the first immunization and with incomplete Freund's adjuvant (IFA) for the second immunization. All the rabbits were subcutaneouly injected intramuscularly in the limbs with the prepared vaccines. Group A were separately immunized with 1 mL recombinant antigen (2×109 cell). Group B was immunized with the C-strain only at day 7 as a positive control. Group C was the negative control and immunized with 0.9% NaCl.
-
The T-lymphocyte proliferation assay was per-formed with MTT dye assay. Briefly, the lymphocytes were isolated according to standard protocol by gradient centrifugation and the buoyant cells were washed three times in RPMI 1640 medium with 10% FBS. MTT dye assay was performed with 96-well flat-bottomed plates. T-lymphocytes (2×107cells/mL) mixed with 100 µL PHA (10µg/mL) were added into the wells. The plates were incubated at 37℃ in a humid atmosphere with 5% CO2. After 45 h, 20µL of MTT solution(5µg/mL) were added to each well and incubated for 3 h. 200µL of a DMSO working solution (192µL DMSO with 8µL 1N HCl) were added to each well for 15min, and then the absorbance was evaluated in an ELISA reader at A570 with a A630 reference. The stimulation index (SI) was calculated based on the following formula: SI=the absorbance value for mitogen-cultures divided by the absorbance value for non-stimulated cultures.
-
Rabbit sera were prepared at 0 (pre-bleed), 28, 42 and 56 days (after the second and the final booster) and also at the termination of the experiment. The sera were tested using the IDXX CSFV Antibody ELISA Kit. The particular procedure was carried out following the kit handbook.
-
The C-strain was diluted with 0.9% NaCl to 200 FD min/mL (FD min, minimal dose to induce fever in SPF rabbits). All the rabbits were inoculated in-travenously with dose of 100 FD min C-strain on the 14th day after the second immunization (3). The ambient temperatures, rectal temperatures and symp-toms were recorded every 12 h during the experiment.
Materials and Methods
Experimental animals
Cell culture and CSF virus
Construction of recombinant plasmid
Production of artificial virus
Target cells infection and selection
Screening for E2 gene in vitro expression
Western blot analysis
Immunization procedures
T-lymphocyte proliferation assay
Detection of anti-CSFV antibodies of vaccinated animals
Virus and experimental inoculation
-
In order to demonstrate appropriate expression of the CSFV E2 proteins, infected PK15 cells were detected by IFA, Western blot, ELISA and PCR. The analysis of IFA demonstrated that CSFV E2 protein was detected in PK15 cells infected with artificial retrovirus (Fig. 1A), which form specific fluorescence, while the blank cell control (Fig. 1B) with the same cells did not show any fluorescence emission.
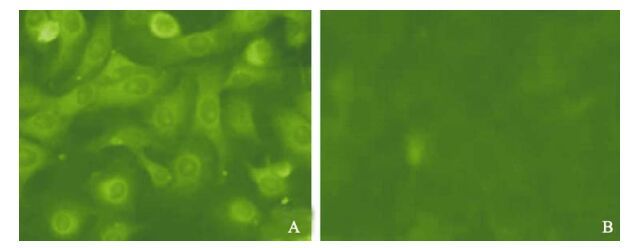
Figure 1. Expression of the E2 gene in PK-15 cells detected by fluorescent antibody. PK15 cells were analyzed for expression of CSFV E2 protein by IFAT. A: The screened positive cells. B: Normal PK15 cell control.
For detecting the activity of expressed E2 protein in vitro, the medium of the infected cells was harvested and analyzed by Western blot and ELISA. As seen in Fig. 2, the picture revealed a major band of 26 kDa in cells infected with"artificial retrovirus", whereas no E2 protein band was found in blank cells. In ELISA assay (as show in Table 1), E2 protein was expressed in PK15-E2 cell culture and culture supernatant according the ELISA kit assessment standard.
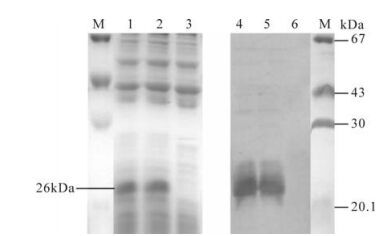
Figure 2. Western blot used to detect the activity of CSFV E2 protein in the infected cells. M, Protein marker; 1 and 2, SDS-PAGE detection of screened positive PK15-E2 cell lysate; 3 SDS-PAGE detection of negative PK15 cell lysate; 4 and 5, Western-Blot detection of screened positive PK15 cell lysate; 6, Western-Blot detection of negative PK15 cell lysate.

Table 1. E2 protein of cell culture detected by ELISA
The CSFV E2 gene was amplified from infected PK15-E2 cells, and a 0.5 kb DNA fragment (using E2-f and E2-r as the primers) was visible (Fig. 3). The fragment was sequenced to confirm its stability and reliability throughout the entire process.

Figure 3. Genetic stability of E2 in infected cells. M, DL2000 Marker. 1, PCR identification of infected 1st generation cells; 2, PCR identification of infected 10th generation cells; 3, PCR identification of infected 20th generation cells; 4, PCR identification of infected 30th generation cells; 5, Blank cell control.
-
In groups A and B, rabbits showed a T-lymphocyte proliferation response (Fig. 4). But none of animals in groups C showed a T-lymphocyte response. Rabbits of group A vaccinated with recombinant antigen alone developed an enhanced response after the second vaccination. Analysis of T-lymphocyte proliferation response with software of biological statistics showed that there was no significant difference between groups A and B, but there was a significant difference between groups A and group C.
-
The CSFV specific antibody levels in the sera that were measured by a commercial competitive ELISA are presented in Table. 2. Groups A that were inoculated with PK15-E2 protein were seropositive on day 2 weeks; this result was also observed in group B that was vaccinated with the C strain vaccine. No animals seroconverted in the control group (inoculated with 0.9% NaCl).

Table 2. Detection of CSFV specific antibodies in immunized rabbits by blocking ELISA
-
If rabbits displayed typical fever after neither the first challenge nor the second one, it means that they are not susceptible to the common dose (100 FD min) of C-strain. In this study, the negative control (group C) suffered a typical fever after challenge with C-strain, while the same dose of C-strain was not able to induce typical fever in all rabbits of the test group (group A) or the positive control group (group B) (Table. 3).

Table 3. Challenge results with C-strain.
Expression of E2 gene in PK15 cell
T-lymphocyte proliferation response of rabbits
Antibody response
Challenge results with C-strain
-
In this study, the four antigen domains of the classical swine fever virus glycoprotein E2 gene was engineered successfully into the genome of PK15 cells by use of a retroviral expression system (containing a retroviral expression vector, plasmid pVSV-G and a packaging cell). In this expression system, the genes coding for the viral structural components were stably integrated into the packaging cell line's genome, and the viral particles produced were replication-incom-petent; to some extent, these characteristic enhance the organism safety and enlarge the application of this system.
In order to identify whether the expressed E2 protein could enhance the cellular immune responses in rabbits, the lymphocyte proliferation test, anti-CSFV antibody test and virus challenge were used to evaluate the response. As shown in Fig. 4. A statisti-cally significant difference of lymphocyte prolife-ration in rabbits in group A and B was observed by comparing with the value of control group C. These results indicate that the expressed E2 protein could significantly increase the activation potential of T cells in rabbits. The antibody levels in the sera of E2-immunized rabbits are shown in Table 2, which demonstrates that the expressed E2 protein is likely to exert an effect on T cells, and is associated with an enhancement of anti-CSFV specific antibody levels. We also found that anti-CSFV antibody titers increased after immunization in the experimental positive group while in the control groups it did not. The summary of our results from the challenge test suggests that this immunity and dose would be adequate for induction of protective immunity.
In previous reports, van Rijn et al demonstrated that Classical swine fever virus (CSFV) envelope gly-coprotein E2, containing one structural antigenic unit, protects pigs from a lethal CSFV challenge (4). In a recent report, vaccination with baculovirus based subunit vaccines, expressing CSFV-E2 or the separate antigenic units A or B/C of E2, protected pigs against CSF (10). Furthermore, the E2 subunit vaccine deve-loped recently against CSFV has been considered a potential marker vaccine as a serological discrimi-nation between vaccinated and infected pigs (8). Our studies, which is the first to express antigenic units A, B, C and D of E2 in mammalian cells, showed significant antigenicity and immunogenicity in vitro and in vivo. The wide-range specific antibody and cellular response against CSFV in vaccinated rabbits were dramatically enhanced, which may be attributed to the recombinant protein E2 characteristic with higher protein activity, functional disulfide bridges, or eukaryotic-specific post-translational modification advantages that the mammalian cells expression system has.
The approach described in this study is relevant for the potential use of a recombinant E2 protein as a multi-epitope subunit vaccine and for the possibility of new opportunities for development of a marker vaccine.













 DownLoad:
DownLoad: