-
Like most DNA genome viruses, herpesviruses express, replicate, and package their genomes in the nucleus. They are then presented with the interesting problem of moving an enormous macromolecular assembly (the nucleocapsid) across the inner and outer nuclear membranes to the cytoplasm. The virus solves this problem by using an envelopment/de-envelop-ment shuttle that involves extensive remodeling of the nuclear membrane. The mechanism that the virus uses to accomplish this is interesting from two points of view. First, the nuclear egress process is conserved among herpesviruses both in its appearance and in some of the viral proteins used to accomplish it. At the same time, aspects of the process are unlike anything that the uninfected cell does with the nuclear mem-brane. These considerations suggest that nuclear egress is an extremely attractive target for antiviral therapy. Second, nuclear egress requires the virus to interact with and modify the function of poorly understood cellular structures. This allows us to use the virus as a tool to explore the structure and function of the nuclear lamina and the nuclear envelope.
HTML
-
It has been clear from the earliest morphological descriptions of herpesvirus-infected cells that virus capsids bud into the nuclear envelope to form enveloped particles in the space between the inner and outer nuclear membranes (13, 19, 50, 59, 65, 66, 71). Intermediates in the process included nucleocapsids docked at specialized areas of the inner nuclear membrane, partially-enveloped capsids in which both inner and outer nuclear membranes are curved around the virus capsid, "stalked" intermediates in which the membrane wrapping around the capsid has been completed, but scission of the inner nuclear membrane has not occurred, and completely enveloped capsids in the perinuclear space. De-envelopment intermediates in which the perinuclear virion envelope has fused with the outer nuclear membrane have also been observed (27, 60, 70). EM analysis provides snapshots of the egress process and does not, by itself, indicate the directionality of the processes observed. The consensus of opinion now favors the model originally proposed by Stackpole (70) in which the major pathway of viral egress from the nucleus requires budding of capsids into the inner nuclear membrane, formation of enveloped virions in the perinuclear space, de-envelopment of the capsid by fusion of the envelope with the outer nuclear membrane. In addition to the morphological observations, this model is supported by two lines of evidence that suggest that the perinuclear enveloped virion is a transient intermediate in the egress process. First, viral proteins naturally targeted to the nuclear envelope or viral glycoproteins engineered to be retained in the ER/nuclear envelope are found in perinuclear virions, but not in mature virions, suggesting that the envelope of perinuclear virions must be lost and replaced at some point in the egress pathway (25, 56, 69, 75). Second, the accumulation of perinuclear enveloped virions associated with specific viral mutants, including mutants in proteins of the viral fusion apparatus, suggest that perinuclear virions require a membrane fusion step to advance in the egress pathway (4, 20, 60). Whether envelopment/de-envelopment is the only pathway for nuclear egress is controversial at present. An alternative pathway in which capsids of herpes simplex virus (HSV) and bovine herpesvirus type 1 (BHV-1) exit the nucleus through dilated nuclear pores has been proposed (40, 76), but does not account for much of the genetic and biochemical evidence that favors the envelopment/de-envelopment model (44), and is inconsistent with data showing that the nuclear pores of infected and uninfected cells show similar gating properties with respect to large molecules (30).
Consideration of the consensus model for nuclear egress suggests that the virus has a set of significant problems to solve in order to successfully negotiate this pathway (Fig. 1). Those problems and the research that provides some hints about how the virus solves those problems are summarized here.
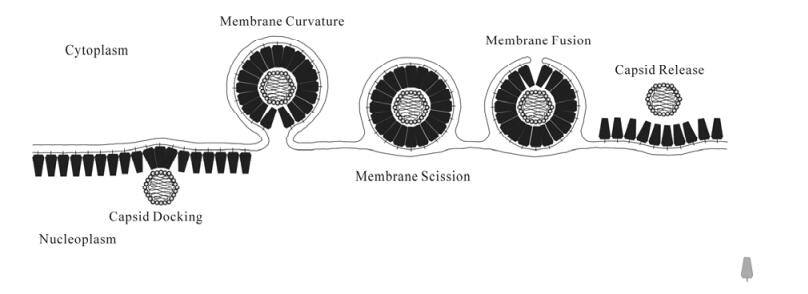
Figure 1. Schematic diagram of a model for nuclear egress. Following local clearing of the nuclear lamina (not shown) DNA-filled capsids are recognized by a membrane-bound envelopment complex (blue polygons). Lateral interactions between envelopment complexes mediate membrane curvature around the capsid. Scission of the inner nuclear membrane results in formation of an enveloped virion in the perinuclear space. Fusion of the virion envelope with the outer nuclear membrane, and disruption of envelopment complex interactions results in release of the capsid into the cytoplasm.
-
Herpes simplex virus pUL34 and pUL31 (the products of the UL34 and UL31 genes) and their homologs in other herpesviruses are required for efficient envelopment at the INM. They are also apparently the only virus-encoded proteins with a conserved function in nuclear egress. HSV-1 pUL34 is a 275 amino acid type Ⅱ transmembrane protein with the bulk of the protein comprising an N-terminal domain that extends into the cytoplasm or nucleo-plasm and a very short (2 a.a.) C-terminal luminal extension. All sequenced herpesvirus genomes have a UL34 gene homolog and all of these homologs in other herpesviruses preserve this general sequence arrangement. UL34 shows no apparent homology to cellular genes. pUL34 function has been most thoroughly studied in HSV and pseudorabies virus (PRV) where the behavior of specific deletion mutants has been characterized. HSV or PRV recombinant mutant viruses that fail to express pUL34 are severely impaired for growth in cell culture and show no evidence of extracellular virus or extranuclear egress intermediates (37, 58). UL34-null infections none-theless show normal virus gene expression, DNA replication and assembly of DNA-containing capsids, suggesting that pUL34 functions specifically in virus nuclear egress. HSV pUL31 is a 306 a.a. soluble protein, homologs of which are also present in all sequenced herpesviruses. HSV-1 pUL34 and pUL31 are targeted specifically to the INM by a mechanism that requires their interaction with each other (55, 56). pUL31 expressed in the absence of pUL34 localizes in the nucleoplasm (25, 55). pUL34 expressed in the absence of pUL31 accumulates in aggregates on the nuclear membrane and on cytoplasmic membranes (37, 55). Expression of pUL31 and pUL34 together, even in the absence of other viral proteins, results in their specific accumulation on the nuclear envelope. The mutual dependence of pUL31 and pUL34 for proper INM targeting is a conserved feature of herpesvirus envelopment (25, 38, 47, 62). Their co-dependence for targeting is correlated with their physical interaction and the sequences in UL31 and UL34 that mediate their interaction and targeting to the INM have been mapped in both proteins (25, 41, 55, 63). Localization of HSV pUL34 to the nuclear membrane results in recruitment and co-localization of other viral and cellular proteins with specific functions in nuclear envelopment, including protein kinase C (PKC) alpha and delta, emerin and the viral protein kinase encoded by the US3 gene (39, 49, 60). Recruitment of PKC isoforms may be a conserved function of pUL34, since it was first observed for the mouse cytomegalovirus (MCMV) homolog (47).
-
Herpesvirus capsids are large structures that are thought to be assembled at discrete sites near, but not at the nuclear membrane (74). While there is no quantitative data that addresses the issue of how far a capsid must move to reach a site of envelopment, there is reason to doubt that random diffusion will suffice. The nucleoplasm of an interphase cell displays different viscosity properties depending upon the size of diffusing structures. Small particles like monomeric proteins diffuse freely through the nucleoplasm and their movement is constrained by a viscosity only slightly greater than that of water; the diffusion of 100 nm microparticles (comparable in size to a herpesvirus capsid), however, is highly constrained (73). Forest et al. visualized the movement of fluorescently-labeled capsids in HSV-infected nuclei and found that most capsids do not move as though diffusing freely through the nucleoplasm, but rather show directed, energy-dependent motion (23). Involvement of actin was suggested by inhibition of active capsid move-ment by latrunculin B, which inhibits actin polymeri-zation by sequestering G-actin. Movement was not, however, inhibited by cytochalasin-D. Association of intranuclear herpesvirus capsids with filamentous actin has also been observed by block-face scanning electron microscopy (21). The significance of actin-mediated movement for virus assembly is not clear, since latrunculin B does not inhibit production of infectious virus, suggesting that it does not signifi-cantly affect nuclear egress (67).
-
The nuclear envelope anchors and is supported by a complex structure called the nuclear lamina. The lamina is composed of a meshwork of intermediate filament family proteins called lamins (1, 28, 77). Lamin proteins are of two types. A-type lamins are encoded by a single gene that is alternatively spliced to give rise to lamins A and C. There are two B-type lamins each encoded by its own gene. The lamin protein meshwork is linked to the INM and to intranuclear proteins by association with integral membrane lamin-associated proteins (LAPs), including emerin, lamin B receptor (LBR), LAP2-β and MAN-1 (reviewed in (31) and (77)). It has been recognized for several years that the nuclear envelop-ment process requires some reorganization of the nuclear lamina. The meshwork of the nuclear lamina is too small to allow passage of capsids through holes in the mesh (1), and physical measurements suggest that the lamin network is quite stiff and resistant to deformation (48). It is likely, therefore, that the lamina must be disrupted in order to allow the nucleocapsid to have access to the INM. An increasing body of literature has documented herpesvirus-dependent changes in nuclear architecture consistent with lamina disruption.
Infection with wild-type virus results in several changes in nuclear architecture, including: (ⅰ) enlar-gement of the nucleus (5, 67); (ⅱ) change in the shape of the nucleus from a smooth ovoid to something that more closely resembles a raisin in contour (5, 67, 68); (ⅲ) changes in the localization of the lamin proteins from a smooth, even lining of the inner nuclear membrane to an uneven distribution showing gross thickening of the lamin layer at some sites and small perforations in the layer at other sites (5, 54, 68); (ⅳ) masking and unmasking of monoclonal antibody epitopes on the lamin proteins that indicate a change in the conformation or associations of the lamin proteins (54). All of these changes require the presence of pUL34 in the infected cell, and at least the changes in lamin distribution and presentation of antibody epitopes also require the presence of pUL31 (5, 54, 67, 68).
The nuclear lamina disassembles at mitosis and reassembles afterwards. Disassembly is associated with phosphorylation of lamins and of LAPs including emerin and LAP2β (11, 14, 17, 18, 22, 29, 51). Herpesviruses appear to adapt this strategy for lamina disruption. As first shown with its MCMV homolog, pUL34 recruits members of the PKC family to the nuclear envelope where they phosphorylate lamin proteins and at least one LAP, emerin. pUS3 is also recruited to the nuclear envelope, where it phosp-horylates lamin A/C proteins and emerin (39, 45, 46). While these activities might be expected to contribute to lamina disruption, pUS3 is not required for for-mation of perforations in the lamin layer. Surpri-singly, deletion of the US3 gene from the virus does not prevent disruption of the lamin layer, but results instead in the formation of large perforations in the lamina, resulting in a characteristic "Swiss cheese" appearance of the lamin layer (5, 39). This result suggests that pUS3 negatively regulates lamina disruption. It may be that the virus has an interest in limiting the disruption of nuclear architecture to the minimum required for efficient nuclear egress.
-
One of the intriguing properties of the nuclear egress system is its selectivity in that nuclear envelop-ment apparently requires completion of the DNA packaging process. In wild-type virus infected cells, empty capsids are only rarely enveloped (57) and a variety of viral mutants that synthesize capsids but fail to complete the DNA packaging process do not efficiently envelop the capsids that are produced (2, 7, 9, 43, 52, 61). DNA-containing herpesvirus capsids (called "C capsids") differ from their empty precursors both in the protein composition and conformation of the virus capsid. DNA packaging results in loss of the scaffolding protein, angulari-zation of the capsid and subsequent recruitment of the pUL35 protein. These changes are also seen, however, in one class of empty capsids called "A capsids." Nonetheless, C capsids are the preferred substrate for nuclear envelopment. Trus et al. have recently reported that A and C capsids can be structurally distinguished by the presence of a C-capsid-specific component (CCSC) positioned around the capsid pentamers and consisting of a complex of the pUL17 and pUL25 proteins (72). In HSV infection, assigning a specific role for pUL17 or pUL25 in nuclear envelopment is problematic since both proteins play important roles in DNA packaging and absence of either protein results in failure to accumulate DNA-containing capsids (43, 61). In PRV infection, however, absence of pUL25 results in production of apparently normal DNA-containing capsids, but these capsids do not egress from the nucleus (35). Presence of the CCSC may "license" a capsid for nuclear envelopment directly or indirectly. pUL25 on the capsid recruits the large tegument protein VP1/2 to the capsid in the nucleus (10). Deletion of the gene encoding VP1/2 is associated with defects in nuclear egress in PRV, but not HSV (16, 42).
-
Tight curvature of membranes is energetically unfavorable, and it is likely that in HSV envelopment, as in other virus budding and vesicle formation processes, membrane curvature is coupled to energetically favo-rable protein-protein interactions. Klupp et al, have shown that over-expression of alphaherpesvirus pUL31 and pUL34 in the absence of other viral proteins can induce formation of small vesicles derived from the INM (34). These data suggest the possibility that, at normal levels of expression, upon recognition of a capsid, these two proteins may interact in a way that induces tight curvature of the INM around the capsid.
-
Virus budding in the cytoplasm, whether at the plasma membrane or into membranes of the secretory apparatus is topologically equivalent to membrane budding into multivesicular bodies (MVBs) (reviewed in (8)). Not surprisingly, some, but not all viruses that bud into cytoplasmic membranes, including herpes-viruses that are undergoing secondary envelopment, recruit proteins of the cellular MVB system for membrane scission at the end of the budding process (6, 8, 12). These cellular proteins have been reported to act at cytoplasmic membranes, but not, so far, at the nuclear membrane. Inhibition of the MVB sorting system by use of dominant negative vps4 protein inhibits secondary, but not primary HSV envelopment, suggesting that nuclear envelopment makes use of some other system (6, 12). There is no topologically analogous process that occurs at the uninfected cell nuclear membrane and no known cellular machinery for accomplishing this. Whether the virus encodes its own scission machinery or recruits an as yet uncharacterized cellular system is a major unanswered question.
-
De-envelopment at the outer nuclear membrane is similar to virus entry at the beginning of infection in that both processes use fusion between the virus envelope and a cellular membrane to deliver a DNA-containing capsid to the cytoplasm. While it would seem economical for the virus to use a common fusion machinery for both initial entry and for de-envelop-ment, things are not so simple. Four virus envelope glycoproteins are required for virus entry at the beginning of infection, gB, gD, gH and gL. gD and gB have receptor-binding activities and gB and the gH/gL heterodimer have been implicated in the membrane fusion event. Deletion of any one of these genes results in failure of the virus to enter cells, but has no significant effect on nuclear egress. In HSV infection, simultaneous deletion of the genes for gB and gH results in accumulation of enveloped virus particles in the space between the inner and outer nuclear mem-branes and decreased, but did not eliminate, produc-tion of mature extracellular virions (20). This result suggests that there may be at least three mechanisms for de-envelopment fusion – one gB-dependent, one gH/gL-dependent, and one dependent upon neither that may use host cell fusion machinery. The degree to which these mechanisms contributes to nuclear egress may be virus-dependent as well since, in PRV, deletion of both gB and gH does not result in accumulation of perinuclear virions (33). In several different alphaher-pesviruses including HSV and PRV, deletion of the US3 gene or elimination of its catalytic activity also results in the accumulation of enveloped virus particles in the space between the inner and outer nuclear membranes, suggesting that the relevant pUS3 substrate plays an important role regardless of the contribution of gB or gH/gL (36, 56, 60, 64).
-
Whatever energetically favorable interactions mediate membrane curvature around the capsid at the inner nuclear membrane must be reversed in order to release the capsid into the cytoplasm. The mechanism of this reversal is unknown, but may involve factors provided by cytoplasmic extracts in an in vitro envelopment system (53).
Recruitment of an envelopment apparatus to the inner nuclear membrane
Movement of capsids from assembly sites to the inner nuclear membrane
Local disruption of the nuclear lamina to allow capsid access to the INM
Recognition of capsids by the envelopment appara-tus at the inner nuclear membrane
Curvature of the inner and outer nuclear mem-branes around the capsid
Scission of the inner nuclear membrane
Fusion of the virion envelope with the outer nuclear membrane
Capsid release into the cytoplasm
-
Evidence accumulated so far is consistent with a model in which events at the nuclear membrane are largely coordinated by an envelopment complex consi-sting of pUL34, pUL31 and pUS3 and their interac-tions with DNA-containing capsids. There are, however, many unanswered questions and genuine puzzles presented by the literature. One of these concersn the role of other viral genes whose mutation results in a nuclear egress phenotype. Deletion or mutation of the UL11, UL20, UL37 and ICP34.5 genes appears to affect the efficiency of nuclear egress in ways that are not obviously tied to their locations in the cell or their other characterized functions (3, 4, 15, 32). In the cases of UL11 and UL20, nuclear egress phenotypes have been observed only with specific deletion viruses that express truncated or fused forms of the proteins whose function may not reflect any activity of the intact protein (24, 26). For UL37 and ICP34.5, it is possible that that they have multiple functions, one of which is modulation of nuclear egress. The nature of those functions has yet to be determined. Interestingly, deletion of UL34 from either PRV or HSV does not completely inhibit the production of viral infectivity, suggesting that there is a minor UL34-independent pathway for nuclear egress (3, 4, 15, 32, 37, 58). The mechanism of this pathway is unknown, but the exis-tence of such a pathway may be consistent with me-chanisms for egress that do not involve envelopment.













 DownLoad:
DownLoad: