-
As insects develop from molt to molt, they become increasingly resistant to infection by baculoviruses (3, 13, 16, 21), but few studies of variation in susceptibility within an instar have been reported (for exceptions see 2, 4, 13]. For example, sensitivity to mortal infection in Lymantria dispar L. (gypsy moth) by L. dispar multiple nucleopolyhedrovirus (LdMNPV) drops dramatically in larvae challenged with virus in the middle of the third or fourth instar (at 24 to 72 h post-molt) (10, 11).
The gypsy moth is an exotic, invasive pest of forests and woody ornamentals in the eastern United States and Canada. LdMNPV is an effective, specific, microbial insecticide against this insect, but it is relatively expensive to produce enough virus to provide effective doses over large areas of forest because in vitro production remains problematic at this time (J. Slavicek, pers. comm.). Larval gypsy moths are most sensitive to lethal infection by LdMNPV immediately after molting (10, 11). They become most resistant in the middle of the instar, and regain some, but not all, of their initial sensitivity at the end of the instar. For example, delivery of a pulse of 325 occlusion bodies (OBs) per larva directly into the anterior midgut produced 88% mortality in newly molted fourth instars but only 29% and 27% mortality in larvae that were orally inoculated at 48 or 72 h post-molt to the fourth instar, respectively (11).
Reports of lepidopteran resistance to baculoviruses within an instar are usually midgut-based, resulting from sloughing of infected midgut cells before the virus has an opportunity to spread systemically (4, 23, 24). In these studies, intrahemocoelic inoculation of larvae produced equivalent mortality regardless of age post-molt within an instar (except for the final instar) (13), indicating that a systemic component to this resistance was ruled out. In contrast, gypsy moths also display developmental resistance to lethal intrahemocoelic inoculation of budded virus (BV) of LdMNPV (10, 11). For example, an LD77 dose of BV delivered intrahemocoelically to newly molted fourth instars produced 29% mortality in larvae that were injected at 48 h post-molt (11).
The objective of this study was to determine if systemic developmental resistance in gypsy moths to LdMNPV is generalizable to other insect viruses, such as Amsacta moorei entomopoxvirus (AMEV). AMEV was originally isolated from the red hairy caterpillar (Amsacta moorei Butler), an arctiid moth from Northern India (17) and was characterized by Granados and Roberts (9) and McCarthy et al. (15). AMEV is a member of the Entomopoxvirinae, a subfamily of the Poxviridae. While LdMNPV is host-specific, AMEV can infect semi-permissive hosts such as the saltmarsh caterpillar Estigmene acrea (Lepidoptera: Arctiidae) (17) and the gypsy moth. Gypsy moth is only considered susceptible to AMEV by intrahemocoelic challenge, unless larvae were fed high doses of virus in conjunction with an optical brightener (18). There may be other hosts permissive to AMEV, but very little is known about the host range of this and other entomopoxviruses (6). AMEV also replicates well in gypsy moth cell lines (5, 8). Despite our attempts to obtain E. acrea or A. moorei to test for developmental resistance within an instar for comparison to L. dispar, we were unable to find a source of these insects for study.
We chose AMEV for this study because similar to baculoviruses, entomopoxviruses (EPVs) are large, double-stranded DNA, insect viruses that can infect the gypsy moth systemically. Also similar to baculoviruses, EPVs initiate infection in midgut cells, but the mechanism of entry remains unknown; entry may occur via fusion with the plasma membrane or by receptor-mediated endocytosis (12). In contrast to baculoviruses, which replicate in the nucleus, EPVs replicate within discrete cyoplasmic foci (viroplasms) in the vicinity of the nucleus (9). Non-occluded, enveloped progeny virions (called intracellular virus or ICV) acquire a second envelope as they bud through the plasma membrane into the insect hemocoel (called extracellular virus or ECV) (8). Thus, EPVs differ from baculoviruses in that the non-occluded form of EPVs is phenotypically the same as the occluded form but are similar to NPVs in producing occlusions that vary considerably in size (5-20 μm in diameter) (1, 12).
To determine if systemic developmental resistance in gypsy moths to LdMNPV is generalizable to other DNA viruses, such as to AMEV, we challenged developmentally-staged cohorts of fourth instar gypsy moths with AMEV orally or intrahemocoelically and compared these results to the comparable time points within the fourth instar in gypsy moths to LdMNPV as reported previously (11).
HTML
-
A stock solution of AMEV ECV in SF 900-Ⅱ tissue culture medium (Invitrogen, Grand Island, NY) was obtained from Marie Becker (University of Florida, Gainesville, FL). We compared viral growth (determined by spheroid formation and appearance of cytopathological effects) in two different subcultures of Ld652Y cells (7) obtained from Becker and Suzanne Thiem (Michigan State University, E. Lansing, MI). Each subculture was tested in both SF 900-Ⅱ supplemented with 9% heat inactivated FBS (Atlanta Biologicals, Norcross, GA) and Tc100 (Sigma-Aldritch) + 10% FBS. We also tested LdEiTA cells (14) in Tc100 +10% FBS and a third subculture of Ld652Y cells in ExCell 420 + 5% FBS (from S. Thiem and James Slavicek, USDA FS, Delaware, OH, respectively). The cells were seeded at ~ 50% confluency in individual wells of a 6-well tissue culture plate, infected with 50 µL of AMEV stock and observed daily using 400x phase contrast microscopy for eight days. In our hands, only the Ld652Ycells from the Michigan State subculture produced occlusions, or displayed significant cytopathology (rounding and detachment from the substrate), and the number of cells with occlusions was ~ 3-4×greater when the cells were grown in Sf 900-Ⅱ + 9% FBS. Thus, AMEV was amplified by passage in LD652Y cells from Michigan State University grown in SF 900-Ⅱ supplemented with 9% FBS. After 7 days, the cells and spheroids were removed by centrifugation at 500×g for 5 min, and the supernatant was stored at 4 ℃. The virus was quantified by plaque assay against LD652Y cells grown in SF 900-Ⅱ + 9% FBS (1.5×106 per 60 mm tissue culture plate) overlaid with 4 mL of a 2:1 solution of 37 ℃ 1.3×SF 900 (Invitrogen, Grand Island, NY) and 4% sea plaque agarose (Cambrex Corporation, East Rutherford, NJ); 300 µL of 2mg/mL MTT was added to the plates 6 or 7 days after infection to increase the contrast of plaques and live cells.
AMEV spheroids were produced by injecting 1 µL (~5 PFUs) of diluted ECV into the hemocoel of newly molted fourth instar gypsy moths using a Pax-100 microapplicator (Burkhard Scientific, Uxbridge, UK) equipped with a 32-gauge stainless steel needle (Popper & Sons, New Hyde Park, NY). Cadavers were collected and the hemolymph inspected microscopically to verify the presence of spheroids. Cadavers were frozen and stored at -80 ℃. Eight to 10g of cadavers were homogenized by hand with a teflon pestle in 2-3 volumes of sterile phosphate buffered saline (PBS) containing 137 mmol/L NaCl, 2.7 mmol/ L KCl, 1.45 mmol/L KH2PO4, 8.1 mmol/L Na2HPO4, pH 6.8. The homogenate was filtered by centrifuging 5 mL aliquots of material through several layers of cheesecloth at 4800×g in a 50 mL conical-bottomed centrifuge tube. The resulting pellet was resuspended in 50 mL of clean sterile PBS and pelleted again at 4 000×g. The pellet was resuspended and centrifuged once more through PBS, and twice through sterile deionized milliQ water. The final pellet was resuspended into 2-3 mL of sterile deionized water. Spheroids were quantified with a hemocytometer.
-
Occlusion bodies (OBs) from the A21 isolate of LdMNPV (20) were amplified in gypsy moth larvae and purified as described previously (11). OBs were maintained as a stock solution in sterile deionized water at 4 ℃ until diluted for bioassays. OBs were quantified with a hemocytometer.
-
L. dispar larvae were reared from surface sterilized eggs obtained from the USDA Insectary (Otis ANGB, MA) as described in Hoover et al. (11) on artificial diet (Southland Products, Lake Village, AR). Newly molted larvae were placed individually in plastic 30 mL cups (Comet Products, Chelmsford, MA) on artificial diet and labeled with the time at which the larvae were to be inoculated. Larvae were designated for inoculation in the fourth instar at 0, 12, 24, 48, 72, or 96 h post-molt (hpm) and are hereafter referred to as 40, 412…496, respectively) (4). At 96 hpm, fourth instars began to exhibit head capsule slippage as pre-molts to the fifth instar.
-
Responses of gypsy moth larvae to oral challenge with AMEV compared with LdMNPV. Oral develop-mental resistance to AMEV within the fourth instar was first examined by per os delivery of spheroids. We inserted a blunt-ended 30-gauge needle between the mandibles and into the anterior midgut to deliver 1 µL of spheroids suspended in 60% glycerol; this inoculation method of gypsy moths was used in our studies of developmental resistance within the third and fourth instars to LdMNPV (11, 10). We also tested AMEV spheroids obtained from Basil Arif (Great Lakes Forestry Centre, Sault Ste. Marie, Ontario, Canada) using the same method. Because these experiments produced very low larval mortality, we re-examined our virus preparation and found that it was made up of a mixture of relatively large and small spheroids. These were separated using a 45/60% w/w sucrose gradient prepared in PBS and centrifuged at 4800×g for 1 h. The small and large particles formed distinct bands at 45% and 60%, respectively. Sucrose was removed from the spheroids by suspension and centrifugation through sterile PBS followed by deionized water at 4000×g. We then tested the larger and smaller spheroids collected from the 45/60% and H2O/45% interfaces, respectively, at a dosage of 2.7×106 spheroids in 40 gypsy moth larvae. Having found that the larger spheroids produced higher mortality, further bioassays were conducted using the spheroids from the 45/60% interface. However, the high concentrations of spheroids caused problems with clogging of the fine-gauge injection needles, so further bioassays were conducted by suspending spheroids in sterile deionized H2O and dispensing 10 µL aliquots onto 1mm thick×5 mm diameter diet discs to deliver a dose of 5×106 spheroids of AMEV per larva. Developmentally-staged larvae (40, 412, 424, 448, and 472) were allowed to feed on the treated diet discs for 12 h, after which time only those that consumed the entire diet disc were retained and returned to plastic cups containing an excess of virus-free artificial diet. We used 30-40 larvae per time point; 15 to 20 larvae were also fed diet discs dosed with deionized water as negative controls.
Mortality and pupation were recorded daily for 28 days at which time all insects had either died or pupated. We could not orally dose larvae at later time points in the fourth instar because at 96 h post-molt, most larvae began to exhibit head capsule slippage as premolts and could not feed. The experiment was repeated 5-6 times per time point. Mortalities for each time point for each virus were pooled and compared using one-way ANOVA and Tukey-Kramer HSD (JMP 5.1, SAS Institute).
Previously published results on oral developmental resistance to LdMNPV within the fourth instar used the dosing method of micro-inoculation of a pulse of virus (11). Thus, to permit a direct comparison of developmental resistance in response to oral virus challenge between AMEV and LdMNPV using the same method of virus delivery, we also performed bioassays using 4o and 448 gypsy moths (the most susceptible and most resistant ages to LdMNPV, respectively) using diet discs contaminated with 300 OBs/larva of LdMNPV following the same procedures as described above for AMEV. In this experiment, there were 25 larvae per time point and the experiment was replicated 3 times. Mortalities were compared between time points using one-way ANOVA.
Responses of gypsy moth larvae to intrahemocoelic challenge with AMEV. To test for a systemic component of developmental resistance to AMEV within the fourth instar, bioassay experiments were conducted by injecting ECV directly into the hemocoel. An initial dose response of a log10 dilution series of our viral stock (6.8×10-2 to 6.8×103 PFU) against newly molted (40) larvae produced typical logarithmic response curves (y = 6.9715Ln (x) + 49.105 R2 = 0.7097), which plateaued at 100% mortality at approximately 6.8 PFUs. We tested dosages of 1 and 0.5 PFU/larva, which produced 77 and 44% mortality in 40's, respectively, and injected this into developmentally-staged larvae (0, 12, 24, 48, 72 and 96 h post-molt to the fourth instar), using 30-40 larvae per cohort. Fifteen to 20 larvae were also injected with tissue culture media as negative controls. Mortality and pupation were recorded daily for 28 days at which time all insects had either died or pupated. The experiment was repeated five times per time point at the 1 PFU dose and 2 times per time point at the 0.5 PFU dose. For the higher viral dose, mortalities for each time point were pooled and means compared using ANOVA and Tukey-Kramer HSD using JMP 5.1 (SAS Institute). For the lower viral dose because there were fewer replicates, data for each time point were pooled and a contingency analysis was performed.
Amplification and preparation of AMEV
Amplification and preparation of LdMNPV
Rearing of insects
Bioassays
-
Larval mortality in controls was less than 0.5% in orally dosed insects and less than 2% in larvae in-oculated intrahemocoelically with media only, and thus no adjustments for control mortality in virus treatments were made.
-
In general, larvae were highly resistant to oral challenge with AMEV. Initial per os dose-responses of a log10 dose series using micro-inoculation produced very little mortality (6.7%), even at dosages as high as 1×105 spheroids. Following sucrose gradient separation, we found that a dosage of 2.7×106 of the larger spheroids produced 43% mortality in 40 gypsy moth larvae, compared with 16.7% at the same dosage of smaller spheroids. So subsequent bioassays of AMEV were conducted using only the larger spheroids. Using this method, mortality was 43 ± 6.9% in 40 larvae, but were significantly lower (2.4-fold) in 424, 448 and 472 larvae (Fig. 1A), indicating marked midgut-based developmental resistance.
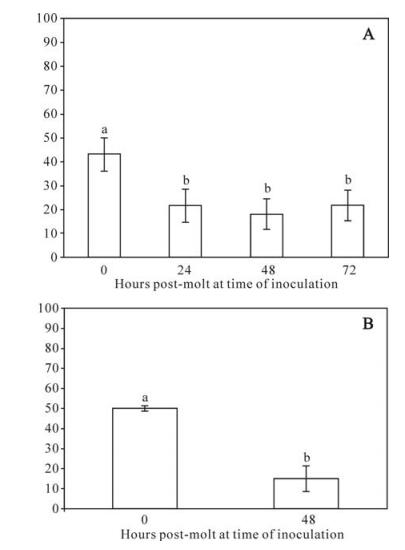
Figure 1. Mean percentage mortality of gypsy moths as a function of age post-molt to the fourth instar to oral inoculation by diet surface contamination with (A) 5×106 spheroids of AMEV or (B) 300 OBs of LdMNPV. For all figures error bars = standard error of the mean. Bars headed by different letters were significantly different at the P < 0.05 level. A: There were significant differences among replicates and between time points, so both effects were included in the analysis. Two-way ANOVA: F7, 650 = 10.8, P < 0.0001. Effects tests: Hours post-molt F = 11.7, df = 3, P < 0.0001; Replicate F = 8.8, df = 4, P < 0.0001. Bars represent the means of 5 replicates of 30-40 larvae per time point. B: There were no significant differences among replicates, so data were pooled for analysis. One-way ANOVA F1, 148 = 33.9, P < 0.0001. Bars represent the means of 3 replicates of 25 larvae per time point.
To permit a more direct comparison of oral developmental resistance of gypsy moths to AMEV versus LdMNPV, we repeated the diet contamination experiment by substituting OBs of LdMNPV for spheroids of AMEV using the most sensitive and most resistance larval ages (40s and 448s, respectively). In these experiments, there was a greater degree of developmental resistance to oral challenge by LdMNPV compared with AMEV (Fig. 1B). At a given dose, mortality was 3.5-fold higher in 40 than in 448 larvae in larvae fed OBs of LdMNPV.
-
In contrast to oral delivery of spheroids, gypsy moths were very susceptible to injection by AMEV. In addition, no developmental resistance was observed to intrahemocoelic inoculation (Fig. 2). At a dose of 1 PFU/larva, mortality was between 77 and 85% regardless of time of inoculation in the fourth instar in response to AMEV with no significant difference in mortality among time points. To determine if developmental resistance to intrahemocoelic virus challenge might occur at a lower dose, we inoculated developmentally-staged larval cohorts with 0.5 PFU/larva. In this case, mortality was between 40 and 48% with no significant difference among times of inoculation post-molt to the fourth instar (Chi-square = 6.2, df = 5, P = 0.2843).

Figure 2. Mean percentage mortality of gypsy moths as a function of age post-molt to the fourth instar to intrahemocoelic challenge with 1 PFU of extracellular virus of AMEV. Bars represent the means of 5 replicates of 30-40 larvae per time point; error bars = standard error of the mean. There were no significant differences in mortality by age, but there were differences among replicates and no interaction between hours post-molt and replicate. One-way ANOVA: F19, 850 = 6.0, P < 0.0001. Effects tests: hours post-molt F = 1.4, df = 5, P = 0.2355, Replicate F = 11.9, df = 4, P < 0.0001.
Responses of gypsy moth larvae to oral challenge with AMEV compared with LdMNPV
Responses of gypsy moth larvae to intrahemocoelic challenge with AMEV
-
Resistance in gypsy moth to oral challenge with AMEV was consistent with a previous report in which no mortality was observed in second instar gypsy moths even at concentration of 106 occlusions by diet surface contamination (18); however, the addition of an optical brightener to the virus preparation rendered second instar gypsy moths susceptible to AMEV with an LC50 of about 6×105 occlusions. In our study, 43% mortality was observed in newly molted fourth instars and 20% mortality in mid-instars, using only the large spheroids of AMEV. These results suggest that the large spheroids are considerably more infectious than smaller spheroids.
Although gypsy moths were highly susceptible to AMEV by intrahemocoelic inoculation, larvae did not show evidence of systemic developmental resistance within the fourth instar to this virus, which is in contrast to systemic developmental resistance in gypsy moths to LdMNPV (10, 11). It is possible that gypsy moth never evolved a specific systemic defense mechanism against poxviruses because the barriers against oral infection are so profound. Since the gypsy moth (a lymantriid ranging from Western Europe East to Japan) is neither closely related to, nor sympatric with, the natural host of AMEV (an arctiid native to the Indian subcontinent), there has probably been little selection pressure to evolve specific resistance against AMEV. Conversely, AMEV should be under little selection pressure to evolve specific mechanisms to counter developmental resistance in L. dispar. Thus, lack of systemic developmental resistance to AMEV suggests that this form of resistance is either specific to LdMNPV, representing a co-evolved interaction; or, once AMEV reaches the hemocoel, it is capable of overwhelming or bypassing host defenses that are normally sufficient to counter infection by LdMNPV.
Unfortunately, specificity of developmental resistance within an instar in L. dispar to LdMNPV could only be tested among insect viruses using AMEV for comparison. To our knowledge, the only other virus pathogenic to gypsy moth is a cypovirus (19), a dsRNA virus that only infects the midgut of its hosts (22).













 DownLoad:
DownLoad: