-
Classical swine fever (CSF) is a highly contagious disease caused by the CSF virus (CSFV) that has serious impact on the pig industry worldwide. The virus belongs to the genus pestivirus of Flaviviridae family along with bovine viral diarrhoea virus (BVDV) [9].
Detection of virus-specific antibodies is a prerequisite for epidemiological surveys in tracing the spread of the virus and for monitoring in an eradication program. Traditional indirect hemagglutination (IHA) tests, using crude or purified antigens of CSFV, have been extensively employed not only for the most widely field diagnosis of CSFV infection but for assessing the kinetics of humoral immune responses in the vaccinated pigs in China. However, routine IHA examination can not distinguish between a case of CSF infected by CSFV and a case of BVDV because there are high homologies in antigen genes between CSFV and BVDV[6, 9].
Epitope mapping has revealed that the 9-amino acid (aa) sequence TAVSPTTLR between 829 and 837 aa of E2 is a major antigenic epitope. This is highly conserved in all CSFV isolates but not in BVDV, and is therefore CSFV-specific[3, 10]. In this study, a poly-peptide consisting of four copies of 20-aa peptide containing this 9-aa epitope was highly expressed in the host cell E. coli and identified by western blot analysis, and an indirect assay using the epitope peptide as antigen was established and compared with a conventional crude complete antigen IHA test.
HTML
-
E. coli JM109 Competent Cells, pGEX-4T-1 vector, pMD 18-T simple vector, positive and negative sera to CSFV were all kept in the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute. The sera against BVDV were purchased from the China Institute of Veterinary Drug Control (Beijing, China).
Glutathione Sepharose 4FF was purchased from Xinjingke Biotechnology Co., Ltd (Beijing, China); BamHⅠ, XhoⅠ, IPTG and penicillin were all purchased from Sangon (Shanghai, China); rabbit against swine HRP-IgG (H+L) was purchased from BioDov-Tech (Beijing, China); DAB was purchased from Amresco (Massachusetts, USA); Plasmid purification kit and agarose gel DNA purification kit were purchased from TaKaRa Biotechnology (Dalian) Co.; CSFV IHA kit was supplied by Lanzhou Veterinary Research Institute.
-
This fragment was synthesized according to a previous report[4]. A 60 bp fragment located at the N-terminal at position 2 846 to 2 905 in the CSFV genome sequence (gi:71084281) was chosen as the antigenic epitope. This region is highly conserved in all CSFV isolates but not in BVDV. The corresponding quadruple amino acid (aa) sequence of the 60 bp gene fragment was spliced by a linker peptide with 4 aa (–SPGS-). Then in order to be expressed more easily, a part of intranucleotide bases of the corresponding amino acid (aa) was changed artificially based on a rule of synonymous codon. The 288 bp nucleotide fragment containing the quadruple 60bp antigenic epitope fragments and BamH I and Xho I sites was synthesized artificially by the TaKaRa Biotechnology (Dalian) Co., Ltd (Fig. 1)
-
The synthesized gene fragment was cloned in frame into the BamH I and Xho I restriction sites of the plasmid pGEX-4T-1 and transformed into E coli strain JM109, following the standard procedure for DNA manipulation. A clone containing the target DNA in the correct orientation was designated pGEX-4E. The positive single clone described above was cultured in 5mL LB medium containing 100 μg/mL Amp overnight at 37℃ with 230 r/min, and 1mL cultures were inoculated with 1000mL LB medium and incubated at 37℃ with 230 r/min until an OD600 value reached 0.6-1.0. The cultures were induced for 4 h at 37℃ with 0.3 mmol/L IPTG. At the same time, pGEX-4T-1 was transformed into JM109 and induced according to the above conditions.
A volume of 1 000 mL cell cultures was centrifuged for 10 min at 5 000×g, and the pellets harvested were resuspended with buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4, 1 mmol/L EDTA, pH 8.0) after being washed one time with PBS (pH 7.4), then lysozyme was added to a final concentration of 100 μg/mL. The cells were then broken up by ultrasound and centrifuged at 4℃ for 15 min at 12 000×g, and the supernatant was collected. The target proteins in supernatant were both analyzed by SDS-PAGE. The recombinant strain JM109/pGEX-4T-1 was processed in the same manner.
The soluble target protein and GST protein in the supernatant were purified with Glutathione Sepharose 4FF by affinity column chromatography. The purified solution protein was filtered through a 0.45 nm filter. The purity and concentration of the protein were confirmed by measuring OD260 and OD280 values. Finally, the recombinant protein activity was analyzed by Western blot [5].
-
A checkerboard titration was performed to optimize the working dilution of the coating antigen, serum and horseradish peroxidase labeled rabbit-anti-bovine IgG (HRP-IgG) on a 96 well ELISA plate. Antigen dilutions were 1:10, 1:20, 1:40, 1:80, 1:160 and 1:320 and serum dilutions were 1:10, 1:20, 1:40 and 1:80 respectively. The dilutions that gave the maximum difference in absorbance at 490 nm between positive and negative serum (P/N) were selected for testing the serum samples on a larger scale. Test sera also included reference controls such as positive, negative and blank samples. At the same time, the reaction temperature, time and other conditions were optimised by the index of P/N value. After optimization above, the indirect ELISA was carried out [1].
-
In total, 110 serum samples, including the 80 positive and 30 negative sera as determined by CSFV IHA kit and the standard negative control, were tested to obtain ODtest serum/ODnegative control (P/N) values. The cut-off value was determined by analysis of a receiver operating characteristic (ROC) curve based on the 110 P/N values[2].
-
A total of 16 sera were selected to validate the test and evaluate the repeatability of the assay. For each sample, the coefficient of variation (CV) was calculated between plates (inter-assay variation) and within the same plate (intra-assay variation). Each sample was tested in four different plates on different occasions to determine the inter-assay CV and four replicates within each plate were used to calculate the intra-assay CV.
-
There were 5 positive sera to BVDV were tested by the indirect ELISA to assess the degree of assay cross-reactivity.
-
In order to determine whether the possible presence of antibodies directed towards GST in sera might produce false results, the GST protein was coated to detect 80 positive and 30 negative sera to CSFV.
Strains, sera and reagents
Design and manual synthesis of the quadruple epitope gene fragment
Recombinant protein expression, purification and Western blot analysis
Development of the indirect ELISA
Determination of cut-off value
Repeatability of the assay
Assessment of cross-reactivity
Indirect ELISA with GST protein antigen
-
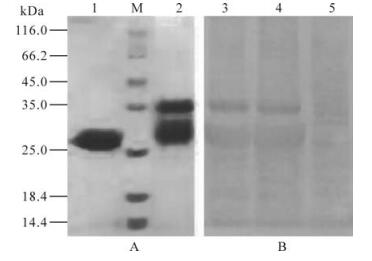
The purified soluble protein was fused GST protein 28.2 kDa -36.12 kDa in size, consistent with the estimated size of 26.0 kDa and could be observed by SDS-PAGE analysis (Fig. 2A). Western blot analysis revealed the band of the expressed protein was between 28.2 kDa -36.12 kDa in size but no band corresponding to the GST protein was observed (Fig. 2B), indicating that the target protein could react with anti-CSFV serum.
-
By checkerboard titration tests, the optical density (OD) value gave the maximum difference between the positive serum and negative serum (P/N value of 2.981) when the dilutions of antigen and serum were 1:160 and 1:20 respectively. So the final concentration of coating antigen was 0.4 μg/well by calculation, and the optimal dilutions of the serum and HRP-IgG were 1:20 and 1: 2000 respectively.
-
Receiver operating characteristic curve analysis determined the cut-off value at 1.93. A serum sample was considered positive if its P/N value was ≥ 1.93. At this value, the highest efficiency of sensitivity and specificity was achieved. With this value, 2 of the 80 positive samples determined by IHA were negative by the indirect ELISA, and 1 of the 30 negative sera by IHA was tested positive by the indirect ELISA. So the ELISA gave 97.5% (78/80) sensitivity and 96.7 (29/30) specificity respectively.
-
For the 16 selected sera, the inter-assay CVs ranged from 2.3% to 6.8%, and the intra-assay CV ranged from 1.8% to 5.6% (Table 1).

Table 1. The inter-and intra-assay coefficients of variation (CV) obtained from assessment of 16 sera.
-
There was no evidence of cross-reactivity with known positive sera to BVDV as all gave values below the defined cut-off point (Table 2).

Table 2. Details of cross-reactivity assessment using samples serologically positive for BVDV.
-
The results showed that the P/N values of the 110 samples were all less than 1.3 and no one sample was positive.
Recombinant protein expression, purification and Western blot analysis
Development of the indirect ELISA
Determination of cut-off value
Repeatability of the assay
Assessment of cross-reactivity
The indirect ELISA with GST protein antigen
-
Anti-BVDV antibodies exist in some serum samples from pigs because BVDV can infect pigs [6]. BVDV and CSFV have high homologies in antigen genes, so anti-BVDV antibodies have cross-reactivity with CSFV, which disturbs the monitoring for antibodies titers against CSFV. In this study, a conserved and specific quadruple antigenic epitope of CSFV E2 glycoprotein was expressed successfully in E. coli. An indirect ELISA was established using the target protein as antigen for detecting anti-CSFV antibodies, and the antigen had no cross-reactivity with anti-BVDV sera in the ELISA assay.
The expressed protein was a fused GST protein 28.2 kDa-36.12 kDa in size (compared to a predicted size of 26.0 kDa) estimated by SDS-PAGE and Western blot analysis, which revealed that the target protein included 1-4 of the original antigenic epitopes. If we select a better linker peptide, the quadruple antigenic epitope probably could be expressed completely in E.coli.
In this test, the fusion recombinant protein including GST was used as antigen, however the possible presence of antibodies directed towards GST in sera might be a problem, which could produce false positives. In order to determine whether this was a problem, the GST protein was coated with 0.4 μg/well to detect 80 CSFV-positive sera and 30 CSFV-negative sera, the results showed that the P/N values of the samples were all less than 1.3 and no one sample was positive. It revealed that the use of fusion protein without cleavage of GST as an antigen in this test might not bring false results.
Crucial to establishing an indirect ELISA are the differences between individual sera due to varying antibody titres, leading to potential false-negative or false-positive results [1, 7, 8]. To overcome this, we used a serial dilution ELISA with the optimal cut-off value derived from a ROC curve based on 110 P/N values. With this cut-off value, the assay exhibited a high degree of specificity and sensitivity. Furthermore, since the inter-assay and intra-assay CVs were ≤6.8% and ≤5.6% respectively, it could be concluded that the assay had good repeatability and could be used in a practical context.
In conclusion, this ELISA provided an alternative, inexpensive and rapid serological detection method that would be suitable for screening for anti-CSFV antibodies titers on a large scale.














 DownLoad:
DownLoad: