-
Grass carp reovirus (GCRV), a tentative member of family Reoviridae, is the major pathogenic factor which causes acute infection to aquatic animals[20]. The virus has a genome composed of 11 double stranded RNA (dsRNA) encoded 12 proteins. Of all the proteins, seven viral proteins (VP1-VP7) form the virion, the remaining five proteins, or nonstructural proteins, are not presented in mature virion but can be detected in infected cells[4, 6]. GCRV, also named as Grass carp hemorrhagic virus (GCHV), was first isolated in China during an acute outbreak of disease which occured in south freshwater culture areas in 1983. It is commonly regarded as the most pathogenic agent among all the isolates of aquareovirus reported to date[19]. Therefore, studies focusing on the protein function of GCRV will have a great contribution to elucidate the virus pathogenesis in general[11].
Although distinct from the viral structural proteins, reovirus non-structural proteins have indispensable roles in virus infection and assembly. Previous studies of the Mammalian orthoreoviruses (MRV) has indicated that the nonstructural protein σNS, together with another nonstructural protein μNS and protein σ3, can associate with mRNA molecules to form single stranded RNA-containing complexes (ssRCCs)[4, 9], Some investigations have implicated that σNS is a minimal viral component required to form viral inclusions with μNS, which then recruits other reovirus proteins and RNA to initiate viral genome replication[4].How the non-structural proteins, such as μNS and σNS, are interacting and affecting each other during viral assembly and replication is still unclear. Therefore, further studies on these proteins is necessary to revealing their functions during the viral life cycle.
Similar to other members of the Reoviridae family, GCRV is an icosahedral particle with a diameter of about 800 ,composing a core and outer capsid shell[6]. The virus replicates well in the CIK (Ctenopharyngodon idellus kidney) cell line at 25− 30℃ and can produce a large syncytia in its sensitive cells[10, 25]. Among the seven proposed genera (A to G) of aquareoviruses[22], GCRV has been characterized the best so far. Sequence and homology alignment analyses indicate that there is great similarities with Mammalian reovirus (MRV) at the protein level[2]. Many investigations have been made on the structure proteins of GCRV[11, 12, 23], but less is known regarding the characteristics of the non-structure proteins. To understand the molecular basis of non structural protein NS38, a full frame protein was expressed in E.coli for the first time. Our results indicated that NS38 has a better expression at 28oC with IPTG induction. In addition, it was shown that the NS38 protein of GCRV can not be detected in GCRV infected cells, indicating it is not a component of the viral structural protein assembly. The result provided in this experiment will lay a foundation for further investigation of the function of NS38 during viral assembly and replication.
HTML
-
CIK (Ctenopharyngodon idellus kidney) cell line, which has been established by Zuo et al. for many years[25], was prepared for proliferating Grass carp reovirus (GCRV). GCRV 873 stain was isolated from Shaoyang, Hunan province in China and maintained in the author's laboratory. The methods related to cell culture and virus infection and replication are as described previously[10].
-
Sequence analysis of GCRV segment 9 was performed using the local-BLAST and Clustal-W programs implemented in the DNATools package. Analysis of protein hydrophobicity profiles and secondary structures were carried out by using the Protean tool of the DNAStar software package[15].
-
The amplification of GCRV Segment 9 gene was carried out by standard RT-PCR. Two primers were designed based on GenBank sequence (AF403395). The pair of primers contained specific restriction enzyme digestion sites according to the multiple cloning sites supplied by the expression vector pRSET-A. The sense primer was: 5'-CAT
$\underline {{\rm{GGATCC}}} $ TACCGATTGACAACCATC-3', the bases underlined are the enzyme digestion site of Bam H I; the anti-sense primer was: 5'-GCT$\underline {{\rm{GAATTC}}} $ ATAGCT CAGAGCGGCATG-3', the bases underlined are the enzyme digestion site of Eco R I. The conditions for thermal cycling parameters were as follows: one cycle of denaturation (94 ℃, 3min) followed by 35 cycles of denaturation (94 ℃, 30 sec), annealing (52 ℃, 1 min) and extension (72 ℃, 1 min and 30 sec). Final extension step was at 72 ℃ for 10 min.After separating and purifying the RT-PCR amplified GCRV S9 segment from the agarose gels using DNA gel extraction and PCR clean-up kits (Hangzhou, China), the S9 segment was then ligated into pRSET-A vector (Invitrogen, Carlsbad, USA) based on the specific matching enzyme digest sites. The ligated products were transformed into DH5 α cells for recombinant plasmid screening. Recombinant clones were confirmed by both restriction enzyme digestion and PCR amplification, which were also confirmed by sequencing (Invitrogen Biotechnology Inc, Shanghai, China).
Identified positive recombinants named as pR/GCRVS9 were transformed into BL21(DE3) pLysS cells (Invitrogen, Carlsbad, USA) for further expression analysis. The positive bacteria were grown in SOB medium with shaking, and then inducing cultivation was performed with 1mmol/L IPTG until the OD600 reached~0.5. To consider optimum conditions for expressing the protein[9], the temperature for inducing culture was set up at 37℃ and 28℃ respectively. After being induced by IPTG for 1h, 3h and 5h, expressed bacteria were collected. Prepared supernatant and pellet of cell lysate samples were stored at −20℃ for further use.
-
Antiserum was generated by immunizing New Zealand white rabbits with a mixed emulsion of 450µg purified NS38 antigen and Freund's complete adjuvant (FCA). The first injection was conducted subcutaneously and two intramuscular booster injections were conducted at intervals of two weeks using the same method. Ten days after the third immunization, the immune rabbits were bled, and separated sera were stored at −30 ℃ for later use. The titer of prepared NS38 antibody was tested utilizing the ELISA method.
-
Samples of expressed supernatant and pellet of cell lysate as well as other testing proteins were resuspended in 2×SDS-PAGE loading buffer, and then subjected to SDS-PAGE. Proteins were visualized by using Coomassie blue R-250 (Sigma, USA). His-tag antibodies (mouse) and rabbit anti-NS38 serum were used to detect the expression of recombinant protein by western blotting analysis. Briefly, tested protein samples were subjected to 10% SDS-PAGE[16], and then were transferred to a PVDF (polyvinylidene Fluoride) transfer membrane by using Semi-dry transfer cell following the instrument's instruction (Bio-Rad). After incubating with first and secondary antibody, the reacted PVDF membrane was developed by using AP substrate solution (NBT/BCIP).
Cell and Virus
Genome structural analysis of GCRV S9 segment
Construction and expression of recombinant plasmid
Preparation of antiserum against GCRV NS38 protein
SDS-PAGE and Western blotting Analysis
-
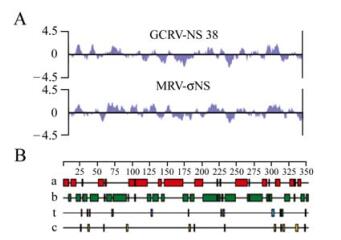
The protein NS38 coded by GCRV s9 segment consists of 352 amino acids with a molecular mass of about 38kDa. Blast analyses suggested that there is about 23% similarities between NS38 and sNS of MRV at the protein level. secondary structure analysis of NS38 (Fig. 1), predicted about 40% of the amino acid residues formed alpha helices, and 49% amino acid residues formed beta sheets. The remaining regions were formed turned and coiled structures. It has ben reported that the sNS protein in MRV isolated from infected cells is able to bind to ssRNA[13]. The strong similarity in the hydrophilicities for the two proteins, suggests NS38 may play a similar role to sNS in the viral life cycle. However, there is a less predicted alpha helix conformation in sNS in MRV, indicating there are some structural dissimilarities between the NS38 and sNS proteins which may be a consequence of the different host cells of MRV and GCRV.
-
To analyze the NS38 protein, we first amplified the 1.1kb GCRV S9 segment using RT-PCR, which was performed by using the selected primer pairs as indicated in the Material and Methods section. The PCR product is shown in Fig. 2A, and is well matched with its predicted value.The pRSET-A vector containing a T7 promoter was chosen for constructing a recombinant plasmid. Its N-terminal polyhistidine makes it convenient for further purification. The amplified S9 fragment and pRSET-A vector were digested with Bam H I and Eco R I respectively, and then ligated by T4 DNA ligase overnight. After transformation, positive plasmid pR/GCRV-S9 was identified by both enzyme digestion and PCR amplification as shown in Fig. 2B, which shows the values correspond well with their predicted size. The recombinants also confirmed by sequence analysis (data not shown).
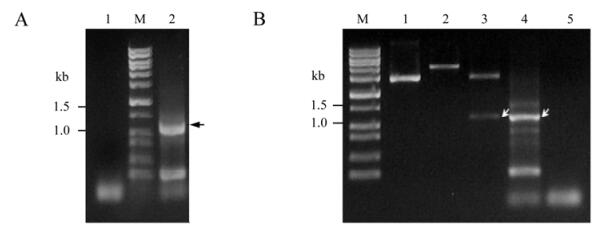
Figure 2. RT-PCR amplification of S9 segment and Identification of its recombinant plasmid. A : RT-PCR amplification of S9 segment. M, DNA mark Ⅲ; 1, Negative control using ddH2O as template (~1.1kb); 2, Positive amplification B : Analysis of recombinant plasmid. M, DNA marker Ⅲ; 1, pRSET vector; 2, Recombinant plasmid pR/GCRV-S9 digested with EcoR I; 3, pR/GCRV-S9 digested with BamH I+EcoR I; 4, Amplified PCR product of plasmid pR/GCRV-S9 (~1.1kb); 5, PCR product of pRSET-A vector. Arrows indicated gene S9 segment.
-
To investigate and analyze recombinant expressed protein, the constructed recombinant plasmid pR/ GCRV S9 was transformed intoBL21(DE3) plysS cells. We first determined expression by using 1mmol/L IPTG for induction at 37℃ for 1, 3, 5 h respectively (Fig.3A). To confirm the expressed product was the fusion protein of interest, Western blot analysis utilizing his-tag antibody was performed. The expressed his-tag fusion protein was about 41kDa, which is consistent with the predicted value. In addition, no obvious band appeared in the cell lysate supernatant samples. The result is shown in Fig.3A' and clearly indicates that the expressed protein appeared in insoluble form and could link to anti-his-tag antibody, suggesting that the expressed NS38 is a his-tag fusion protein.
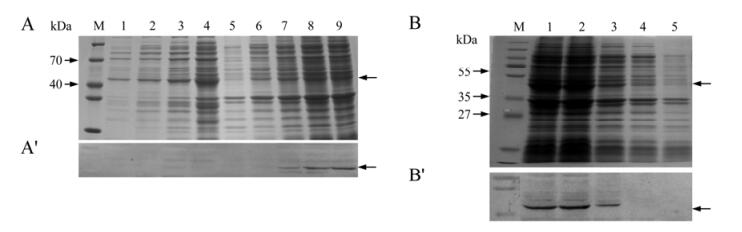
Figure 3. Time course expression of NS38. A: SDS-PAGE analysis of Induced expression at 37℃. M, Standard protein marker; Lane 1, Expressed pRSET empty vector as negative control induced for 3 h by IPTG (supertant); 2-4, Recombinant NS38 protein expression cell lysate supernatant induced at 37℃ by IPTG for 0, 1, 5 h, respectively; 5, Expressed pRSET empty vector as negative control induced for 3h by IPTG (pellet); 6-9, Recombinant NS38 protein expression cell pellet induced at 37℃ for 0, 1, 3, 5 h, respectively (as arrows indicated). A': Western blot analysis corresponding to above protein samples with anti-his-tag antibody. Lane 1-9 matches lane 1-9 in A. B: SDS-PAGE analysis of induced expression at 28℃. M, pre-stained Standard protein marker; Lane 1-4, Recombinant NS38 protein expression cell pellet induced by IPTG at 28℃ at 5, 3, 1, 0 h, respectively; 5, Expressed pRSET empty vector as negative control induced for 3 h by IPTG. B': Western blot analysis of time course expression corresponding to above protein samples with anti-his-tag antibody. Lane 1-5 matches lane 1-5 in B.
For improving the yield of expression, we further set up time course expression with IPTG induced at 28℃ based on optimum culture temperature for GCRV proliferation[10]. Fig. 3B shows there is improved expression induced by IPTG at 28℃ compared to 37℃[9], and Western blot confirmed the identity of the expressed protein (Fig. 3B'). The result suggests that the recombinant expression of the protein NS38 may correspond to the viral replication econiche in infected cells.
-
In order to perform functional assays of the nonstructural protein of GCRV, the purification of the NS38 protein is of great importance. Because the pRSET vector contains a 6 tandem histidine peptide Tag in its N terminal, we can make full use of its distinctive high affinity to probond resin for protein purification. The purified NS38 protein by using denaturing condition was shown in Fig.4A.
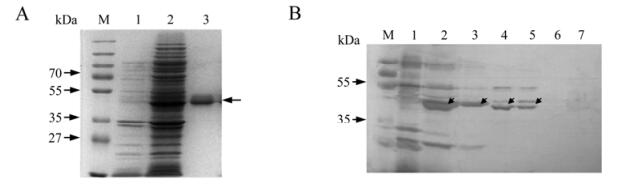
Figure 4. Purification and western blot analysis of expressed protein NS38. A: SDS-PAGE. M, Pre-stained standard protein marker; Lane 1, Expressed empty vector induced 3 h by IPTG; 2, Unpurified expression lysate of protein NS38. 3, Purified and dialyzed expression lysate of NS38 protein. B: Western blot detected utilizing rabbit anti-NS38 antibody. 1, Expressed empty vector induced 3 h by IPTG as negative control; 2 and 3, expressed NS38 protein; 4 and 5, virus infected CIK cell lysate p.i. 24h; 6, Normal CIK cell lysate; 7, Purified viral particles. Diagonal arrows indicate NS38 protein.
Western blotting analysis was conducted to further determining the specificity of the expressed fusion protein and its immunologically binding to the nonstructural protein NS38 during GCRV replication. The result demonstrated that the prepared antibody could specifically bind to the expressed NS38 protein in E. coli . Moreover, the NS38 expressed in GCRV infected cells could be detected by prepared polyclonal rabbit anti-NS38-serum (Fig. 4B). No NS38 protein could be detected in purified GCRV particle, indicating NS38 is a nonstructural protein.
Genome structure analyses of protein NS38
Construction of recombinant plasmid of GCRV Segment 9
Time course expression of recombinant NS38 and western blot analysis
Purification and specificity analysis of expressed protein NS38
-
In this report, we expressed the NS38 protein for the first time and found that the level of expression at 28℃ was much higher than that of inducing expression at 37℃, which is consistent with our previous study on expression of another nonstructural protein (NS80) of GCRV[9]. As previously reported, GCRV replicates well in the CIK (Ctenopharyngodon idellus kidney) cell line between 25-30 ℃[10]; the resulted temperature at 28℃ for recombinant expression of non-structure protein NS38 suggested the expression of nonstructure protein in vitro may correspond well with GCRV replication conditions in infected host cells.
Whereas structural proteins are component of the composed viral particle, non-structural proteins only occur in the virus replication cycle in infected host cells. Therefore it is interesting to note that the yield of recombinant expression of structure protein is much higher than that of whole frame expression of nonstructural protein in our current studies[8, 9, 23]. However, for truncated expression of non-structural protein NS 80C, high level expression can be achieved, suggesting a toxin domain might be located in N-terminal region of NS80. In such a case, to map functional domain of NS38 need to be sonducted in further study.
Earlier studies pointed out that the sNS protein formed small spherical or triangular reovirus-specific particles that played an important role in dsRNA synthesis in infected cells [14]. The interaction among sNS, μsNS and core protein have indispensable role in the formation of viral inclusions as well as recruiting other viral proteins and RNA to combine into complexes for replication and assembly [3, 5, 13, 21, 24]. Understanding the exact mode by which sNS interacts with other proteins in an infected cell should provide additional insight toward elucidating its possible function in mRNA binding and particle assembly in reovirus replication [3, 4, 13]. Compared to reovirus structural protein [7, 17, 18], the molecular mechanism of non-structural protein in the reovirus life cycle is less understood [1]. The result reported in this study provides information for further functional study of NS38 and its interaction with other nonstructural and structural proteins or RNA in dsRNA virus replication and assembly.













 DownLoad:
DownLoad: