-
Influenza viruses are enveloped RNA viruses that belong to the family of Orthomyxoviridae and can cause signiflcant morbidity and mortality in humans through epidemics or pandemics, the latter of which has occurred on three occasions in 1918, 1957, and 1968 [12]. The most common infection, seasonal influenza, is usually a mild, self-limited febrile syndrome, but it can be more severe in infants, the elderly, and immunodeficient persons, in whom it can progress to severe viral pneumonitis or be complicated by bacterial superinfection, leading to pneumonia and sepsis. Occasionally, viruses that have spread from wild birds to domestic poultry, such as highly lethal H5N1 avian influenza that first emerged in Hong Kong in 1997 can also infect humans. More recently, genomic segments of a new H1N1 strain were found to be most closely related to swine influenza strains.
Currently available anti-influenza virus drugs target either the viral M2 ion channel (amantadine and rimantadine) or the viral neuraminidase (oseltamivir and zanamivir) [6]. Because Amantadine is not effective against influenza B viruses and can cause adverse effects, it is not recommended [8, 14-16]. A signiflcant increase in amantadine resistance among H3N2 FLUAV circulating in Asia, Australia, North America, and Europe was noticed in recent antiviral surveillance studies [1, 3, 4, 13]. This raises further concerns about the appropriate use of adamantanamines. Moreover, some of the currently circulating human-pathogenic avian H5N1 viruses in South East Asia [2, 5, 11] and other avian FLUAV subtypes [3]are amantadine-resistant.
Due to the high prevalence of amantadine-resistant viruses, neuraminidase inhibitors (NAI) are the only drugs that are currently considered for antiviral therapy of influenza virus infections. However, the 2007-2008 influenza season in the Northern hemi-sphere has shown a marked increase in the number of H1N1 isolates that are resistant to oseltamivir [7, 9].
Another commonly used antiviral agent is virazole, which is used as positive control in our experiment. However, virazole has many side effects such as epileptic seizures, convulsion, somnolence, sinus bradycardia, sinus arrest, urticaria, anaphylactic shock, hemolytic anemia, aplastic anemia, oligoleukocy-themia, impairment of renal and liver functions, systematic bleeding, DIC and angina abdominis. All of these problems restrict its clinic usage. Thus, with the limited range of current treatments and the threat of a new pandemic, influenza drug development remains a high priority.
Folium Isatidis is derived from the dry leaves of the Cruciferae plant -Isatis indigotica Fort. The plant has long been recognized as a herb in Traditional Chinese Medicine, for treatment of influenza, acute infectious hepatitis, dysentery, acute gastroenteritis, acute pneumonia and so on. Modern research has proved that Folium Isatidis has antibacterial, antiviral, antipyretic, anti-inflammatory properties and can promote immunological response. In this study, we extracted a monomer from Folium Isatidis to demonstrate its anti-influenza virus activity in vivo.
HTML
-
The monomer from Folium Isatidis was provided by the Pharmacy College of Tongji Medical University. It was made up into 0.1g/mL water solutions. After the sterilization under 8 pounds' pressure in 15 minutes, we packed them into small bottles for experiments.
-
Influenza virus strain (A/Yamagata/120/86 H1N1) was donated by Professor Shiro Shigetafrom Department of Microbiology, Fukushima Medical University School of Medicine (Japan). The virus was propagated in the allantoic cavity of 9-11 day old chicken eggs. The allantoic fluid was harvested and the titer of erythrocyte agglutination was above 1:320. The allantoic fluid was stored and divided equally at -80℃ until use.
The MDCK cells were provided by Wuhan University Typical Cell Storage Center (China). The cell lines were grown in DMEM (Hyclone, USA) supplemented with 10% fetal calf serum (Shanli, China), 100 U/mL penicillin, 1% L-glutamine and 100 mg/mL streptomycin. The maintenance solution had the same percentages of ingredients as the culture solution except 2% calf serum.
140×2 mice in equal numbers of male and female Kunming Species mice weighing between 18-22 g were provided by the Laboratory Animal Center in Wuhan University
-
The mice were randomly divided into seven groups. Group 1 to 6 were infected with H1N1 influenza virus. Group 7 was not infected with H1N1 and admini-strated with physiological saline as a normal control. Group l, 2 and 3 were used as negative or positive control groups, which were administrated with 0.5g/kg/d physiological saline, 0.09g/kg/d virazole and 1.5g/kg/d anti-virus oral liquid respectively. Group 4, 5, 6 were used as test groups, which were administrated with 25mg/kg/d, 50mg/kg/d and 75mg/ kg/d dose of the monomer respectively.
-
After the mice were anesthetized with aether, they were infected intranasally with 20 LD50 (6.0 g/kg as have tested) H1N1 0.05 mL every mouse. 2 h later, 0.5mL drug or physiological saline was given to each mouse once a day for 6 d. 12 h after the last administration, the mice were fasted, weighed and sacrificed. Afterwards, the chests were opened to remove the whole lungs which were then washed, dried and weighed. The lung index and the inhibitory rate of lung index were determined by the following formulas:
Lung index=weight of the lung/weight of the mouse×100%;
Inhibition rate of lung index= (lung index virus control –lung index tested)/ lung index virus control ×100%.
-
Lungs were observed by naked eyes. It was found that the pulmonary pathological changes varied amongst the different groups, with the degree of the pathology in the lungs of the drug treated group less severe than the virus control groups. The drug treated groups only exhibited slight lesions and a small number of inflammatory corpuscles in the lungs. Conversely, exudant from the pulmonary alveolus and lung grayish brown consolidation appeared in the virus control groups. Therefore, the extent of lung lesions was graded as follows: "±": the lesion area occupies one fourth or less of the whole lung."+": the lesion area occupies one fourth to half of the whole lung."++" : the lesion area occupies one half to three quarters of the whole lung."+++": the lesion area occupies three quarters to four fifths of the whole lung."++++": the lesion area occupies nearly the whole lung.
-
Three mice lung tissues were randomly selected from each group and homogenized with normal saline (in the ratio lung weight (g) / normal saline (mL) =1/9), centrifuged (4 ℃, 3 000 r/min, 20 min), and the supernatant was collected. Then 20 μL normal saline was added to each well (except for the first well) in a 96-well plate. 40μL test virus suspension was added to the first well, then 20 μL was transferred from the first well to the second well. After mixing, 20 μL mixture was transferred from the second well to the third well, and so on. Thus we did 2x serial dilution across the entire 96 well plate.
After diluting the virus suspension, 20μL 1% chicken red blood cell suspension was added to each well And the solution was mixed and left for 45 minutes at room temperature. Afterwards, blood clotting was observed. We regarded the appearance of influenza virus hemagglutinin "++"(a ring formation of red blood cells at the bottom of the hole surrounded by a small aggregation block) as the highest dilution end point and that dilution multiple of the influenza virus as the hemagglutination titer. Normal saline control (excluding non-specific agglutination of blood cells) and virus control were also used. The inhibitory rate of viral was determined by the following formula:
IV= (HTc-HTt)/HTc×100%, in there, IV was virus inhibitory rate, HTc for hemagglitination titer of virus control, and HTt for hemagglitination titer of tested group.
-
Another 140 mice were randomly divided into seven groups of 20 mice. We orally administered 0.05mL of drug or normal saline to every mouce once a day, for 2 d. 2 h after administration on the second day, we infected the mice with 0.05mL of HIN1 (20 LD50) intranasally under slight anaesthesia, then administered the drugs to the mice for 6 continuous days. Symptoms were observed for 2 weeks and the number of deaths and survival days of animals in each group were recorded. Consequently calculate the ratio of death protection and the ratio of prolonging life according to the formulas below.
Mortality rate = (death rate of virus control-death rate of tested group)/death rate of virus control ×100%
Survival rate = (survival daystested-survival daysvirus control)/ survival daysvirus control ×100%.
-
Statistical analysis was performed with the SPSS 13.0 software package. We use correlation & regression analyze to get the equation of a straight line and coefficient of correlation. The virus inhibitory rate, mortality rate and survival rate of different groups (FI, virazole and antiviral oral liquor)were compared with one-way ANOVA(S-N-K and Dunnett method). P value 0.05 or 0.01 was considered statistically signiflcant. Survival rate was analyzed by the Kaplan-Meier method.
Drug preparation
Virus, Cells and animals
Animal experiment groups for treatment
Lung index
Pulmonary pathological changes
Pulmonary virus hemagglitination titer
Survival rate and death rate
Statistical Analysis.
-
From Table 1, the lung index of low, median, high dosage are significantly lower than the virus control groups, F=9248.750, P < 0.01. In addition, the lung index and its inhibitory rate of the FI high-dose has the similar effect with virazole, P=0.086 > 0.05; but is more effective than the antiviral oral liquor, P < 0.01. And there are obvious dose-effect relationship between dosage (25~75 mg/kg/d) and the inhibitory rate of lung index, P < 0.05.r=-0.997, y=2.183-0.011x.

Table 1. The effect of FI against lung index in influenza mice
-
In Table 2, the numbers of "+++~++++" in FI given group are less and the numbers of "±~++" are more than than those in the virus control group. This indicates that the monomers from Folium Isatidis can cure the pneumonia caused by influenza virus somehow.

Table 2. The effect of FI against pathological changes in influenza mice lung
-
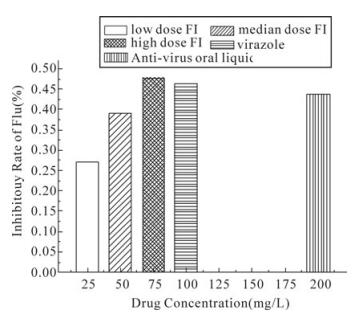
From Table 3, the hemagglutination titers in different dosage FI group are much lower than the virus control group, F=1766.000, P < 0.01. Meanwhile, there is a clear dose-effect relationship between the dosage (25-75 mg/kg/d) and inhibitory rate of the lung index, r=0.997, P=0.014 < 0.05. y=0.964-0.005x. And in Fig. 1 the high-dose FI monomer can decrease the hemagglutination titers more effectively than anti-virus oral liquid (P = 0.002 < 0.01), while the same as virazole. (P = 0.086 > 0.05)

Table 3. The effect of FI against influenza virus hemagglutination titer in mice lung
-
After 4 days' infection, the mice began to reduce their activities, lose their weights and appetite, let their hairs erected, chill, crouch and have various degrees of breathing blister sounds. 5 days after infections they began to die, and this phenomenon continued for 2 weeks.
Some mice were able to gradually return to normal conditions, while some can not and begin to die. We count the number of deaths and calculate the survival days in each group for 14 d in Table 4. (If mice are not dead in 14 days, we regard their survival days as 14 days). And there is an obvious relationship between the dose and death rate. Different dosages of FI could significantly reduce the mortality rate (P < 0.01), extend the living time (P < 0.01). In addition, it was proved that the high-dose group differed little from the virazole group(P > 0.05), and is more effective than antiviral oral liquor in its antiviral activity, P < 0.05.

Table 4. The death protection effect of FI to mice infected by influenza virus
Effect of the effective monomer from Folium Isatidis(FI) to the influenza mice lung index
Effect of FI against pathological changes in influenza mice lung
The effect of FI against influenza virus hemag-glutination titer in mice lung
The death protection effect of FI to the mice infected by influenza virus
-
H1N1 strains of influenza virus infection can cause viral pneumonia in mice and inflammatory exudate can increase lung weight. According to the formular: Lung index=weight of the lung/weight of the mouse× 100%. Hence the more weight the lung has, the higher the lung index will be. Thus the lung index is a useful indicator to evaluate the degree of the lung lesions. When someone's lung has lesions, lung tissue can be filled with pus and other fluid, which makes it difficult for oxygen in the lung's air sacs to reach the bloodstream. The person may have difficulty breathing and have a cough and fever; occasionally, chest or abdominal pain and vomiting are symptoms, too.
In our study, 25-75 mg/kg/d dose continuously administrated for 6 d could significantly decrease their lung indexes (P < 0.01), increase the rate of viral inhibitions (P < 0.01), and reduce the extent of his inflammatory lesions. A dosage of 75mg/kg/d has comparable antiviral effect as virazole (100mg/kg/d), P > 0.05, but is more effective than antiviral oral liquor (200mg/kg/d), P < 0.05.
From the mortality experiments we found that more than 90% of mice died after infection with 20 LD50of H1N1. Different dosages of FI such as 25-75 mg/kg/d could obviously reduce the mortality rate (P < 0.01) and prolong the survival rate (P < 0.01). All this showed that FI monomer reduced mortality rate of mice infected by influenza virus H1N1.
The influenza virus hemagglutinin levels are closely related to number of infectious virus particles, thus the virus hemagglutination titer could reflect the quantities and virulences of the virus to some extent. Therefore, we test hemagglutination titer from the lung tissue suspensions which is the major organ infected by virus. The results showed that the different dosage of the monomer from Folium Isatidis could evidently reduce the lung hemagglutination titer in the mice.
All this confirmed that the monomer from Folium Isatidis could inhibit the proliferation of influenza virus in vivo effectively. In our future work we will perform additional work to gain further insight into its antiviral mechanisms and targets at the molecular biology and molecular pharmacology level.













 DownLoad:
DownLoad: