-
Peste des petits ruminants (PPR) is an acute, febrile, contagious viral disease that causes high morbidity and mortality in sheep and goats and is considered as one of the major constraints in improving the productivity of small ruminants in enzootic areas [20]. Despite strict control measures including statutory regulations along with availability of scores of vaccines and diagnostics, this infection still remains as a constant threat to livestock. Vaccination has become a recommended tool to support eradication efforts and limit the economic losses due to PPR. The only way to control PPR is by the use of a homologous vaccine against the disease. Of late, there are three Vero cell culture based live attenuated PPR vaccines that are available globally (one from an African isolate [7] and two from Indian isolates [19]). The vaccine developed from an African (lineage I) isolate (PPRV strain Nigeria 75/1) in Vero cells was the only commercially available product until recently [6].The vaccine produced by the Indian Veterinary Research Institute (IVRI), Mukteswar, India is being used widely throughout India for the control of PPR [10, 16, 19]. Although this vaccine was found to give a good and long lasting protective immune response [15], it has poor stability at ambient temperatures and requires cold-chain maintenance during transport and storage [16]. Therefore, the vaccines which possess both excellent storage stability and heat resistance capabilities have been in demand.
In general, live attenuated veterinary vaccines suffer serious deterioration in vaccination campaigns due to the difficulty in maintaining cold-chain during the storage and transport of vaccine, which inevitably result in loss of vaccine potency in tropical and subtropical environments and this is one of the major constraints in control of the viral diseases. Thermo-stable vaccines are considered more suitable under tropical field situations wherein the viability of the vaccine viruses will be ensured. In India the control strategy is aimed at vaccination using conventional live attenuated PPR vaccine [16, 19]with sero-monitoring/surveillance by PPR-competitive ELISA [21]. Though these methods are effective, the major problem in mass vaccination and eradication programs lies in the potential failure of these vaccines as they require maintenance of cold chain from production until delivery in the field. In a tropical country, one of the main hurdles in achieving reliable vaccine coverage is lack of a cold-chain infrastructure; hence work was initiated to address the problem of deterioration of vaccine quality under conditions of high temperature exposure, in order to meet the increasing demand as well as need for development of thermo-stable vaccines in India.
Recently, thermo-adapted (Ta) PPR vaccine candidate viruses [Jhansi/2003 (goat origin) and Revati/2006 (sheep origin)] have been developed at the Division of Virology, IVRI, Mukteswar, India [3]. These vaccine viruses were isolated from PPR outbreaks [4] and were subsequently adapted and attenuated (up to 50 passages) gradually in thermo-adapted Vero cells grown at higher temperature (40℃) following the method described by Raut et al. [11], which resulted in two thermo-adapted PPR vaccine candidate viruses (unpublished data) which were then genetically characterized [3]. These vaccines underwent successful in-house trials in both sheep and goats and were found safe (at 105 TCID50/dose); potent (at 103TCID50 or 102TCID50/dose) and efficacious (un-published data). These vaccine strains provided a protective immune response in sheep and goats subsequently challenged with highly virulent PPRV in goats only (unpublished data). Currently, these two Ta PPR vaccines are undergoing field trials for their safety and potency evaluation in sheep and goats in India. This current study was envisaged to compare the efficacy of two extrinsic stabilizers (conventional lactalbumin hydrolysate-sucrose (LS) and the modified stabilizer-stabilizer E) in the induction of thermal protection to the above candidate Ta PPR vaccine viruses in lyophilized form as well as in the reconstituted form with the diluents (1moL/L MgSO4 or 0.85% NaCl) at various temperatures which are normally encountered under field conditions.
HTML
-
Thermo-adapted (Ta) vaccine viruses (PPRV Jhansi/ 2003 and PPRV Revati/2006) in the thermo-adapted (Ta) Vero cell (grown at 40℃) [3] were used in the study. Thermo-adapted Vero cell line available in the National Morbillivirus Referral Laboratory (NMRL), Division of Virology, IVRI, Mukteswar was used for the propagation of vaccine viruses. Thermo-adapted Vero cells between 20-30th passages were propagated in Eagle's minimum essential medium (EMEM) (Sigma, USA) containing 10% fetal bovine serum (FBS) and for maintenance, EMEM with 2% FBS was used for production of vaccine in bulk as well as for virus titrations.
-
Two stabilizers, lactalbumin hydrolysate-sucrose (LS) [16] and stabilizer E-divalent cationic-sugar stabilizer [1]with modifications were used in this study. The LS stabilizer consisted of 5% lactalbumin hydrolysate (LAH) (Difco Laboratories Inc, USA) and 10% sucrose in Hank's balanced salt solution (HBSS), pH 7.2 and the modified stabilizer E contained 30% trehalose dihydrate, 0.02 mol/L L-histidine, 0.02 mol/L L-alanine, 0.02 g/L of CaCl2 and 0.152 g/L of MgSO4 for suspending the viruses before lyophili-zation. The concentrations mentioned here indicate the final strength of each stabilizer in the vaccine preparation.
-
Thermo-adapted Vero cells were seeded into roller culture bottles (1700 cm2) (Corning inc, NY, USA) at a concentration of 2.5×107 cells. Two days after cell seeding, an even, confluent monolayer was produced, and the bottles were immediately infected with vaccine virus at a multiplicity of infection (moi) of 0.01 to 0.05. Infected bottles were incubated at 40℃ in the roller apparatus. After 6-7 days post-infection (dpi) when more than 80-90% cytopathic effect (CPE) was observed, virus was harvested from the infected cells by a cycle of freezing and thawing. To maintain a uniform virus titre, virus harvest from all the roller bottles were pooled after the first thaw and were divided into different aliquots. The aliquots were preserved at -80℃ until used for lyophilization.
-
The viruses were lyophilized in sterile 5 mL capacity vaccine vials using an Edwards Modulyo 4K freeze-dryer. Equal volumes of the vaccine and stabilizer (2X concentration) were mixed. One milliliter of the mixture was dispensed in vaccine vials and partially sealed with vented rubber stoppers. The vaccine vials were kept at -80℃ overnight and lyophilized at a condenser temperature of -60℃ and a vacuum of 0.06 mbar. After 48 h of lyophilization, vials were rubber-stoppered under vacuum, then tightly sealed with an aluminum cap under normal air pressure. A batch of vaccine containing both the different stabilizer formulations were lyophilized simultaneously under identical conditions to compare the quality in terms of residual moisture (RM) and titre loss during lyophilization.
-
Residual moisture of lyophilized vaccine was measured by thermo-gravimetric method as described by Worrall et al. [25]. According to this method, the mean weight of 10 vials from each vaccine batch was taken and the content of each vial was then dried for 20 h at 80℃. After drying, the vials were weighed again and the weight of water lost from the dried vaccine was expressed in percentage.
-
The batch of freeze-dried test vaccine vials having an initial titre of 5.75 log10TCID50/mL for Ta PPR Revati/2006 vaccine stabilized with both stabilizers and initial titre of 5.89 and 5.83 log10TCID50/mL for Ta PPR Jhansi/2003 vaccine stabilized with LS and E stabilizers, respectively were subjected to the thermo-stability study. Sufficient numbers of freeze-dried vials of each vaccine with each stabilizer were exposed at different temperature (25℃, 37℃, 40℃, 42℃ and 45℃) in a thermally controlled incubator /dry oven. The vaccine vials were sampled at various time intervals and temperature schedules. Exposed vaccine vials were sampled on monthly intervals (1, 2 and 3) at 25℃ incubation, on days 3, 5, 7, 10 and 14 for those samples kept at 37℃ incubation; on days 1, 2, 3, 4 and 5 for 40℃ incubation; at 12 h interval up to 48 h for 42℃ incubation and at 8 h interval up to 40 h for 45℃ exposed samples. Exposed samples were reconstituted with 1 mL of serum free EMEM and titrated in thermo-adapted Vero cells. For each exposure, three samples were titrated in triplicate and their average log titer was calculated.
-
The batch of freeze-dried test vaccine vials having an initial titre of 5.5 log10TCID50/mL for Ta PPR Revati/2006 and PPR Jhansi/2003 vaccines stabilized with both stabilizers (both vaccines stabilized with LS, and stabilizer E) were subjected to the thermo-stability study. Freeze-dried vials of each stabilizer were reconstituted with two diluents (1 mol/L MgSO4 and 0.85% NaCl). For each stabilizer, three vials were taken and reconstituted separately with 1mL each of the two diluents and the reconstituted vaccines were then exposed at 4℃, 25℃ and 37℃. Samples from each temperature were taken out at 6 hourly intervals up to 48 h and titrated immediately.
-
After the specific incubation or exposure time, either the re-hydrated freeze-dried vaccines or the reconstituted vaccines were subjected to virus titration. Serial ten-fold dilutions of exposed virus suspension were made immediately in maintenance medium and the viruses were titrated in monolayers of thermo-adapted Vero cells grown in 96-well microtiter plates using four replicates as per dilution (100 µL/well). The plates were incubated in the presence of 5% CO2 for 6 days with a change of maintenance media at every alternative day and cells were observed for cytopathic effects (CPE) regularly under microscope. Virus infectivity was quantified by estimating the 50% tissue culture infectivity doses (TCID50) and end points were calculated as per Reed and Muench [12] (simplified format made in the MS Excel for calculations). After visual observation of PPRV-specific CPE on the sixth day, final reading of the micro titre plates was re-confirmed by cell-ELISA to detect the presence of virus as described earlier [14, 22].
-
The viability of two thermo-adapted viruses at different temperatures over a different time intervals or exposure period was carried out by regression analysis. In order to calculate the shelf-life and half-life of vaccines regression analysis was employed. The shelf-life is defined as the time required to reach 4.5 log10 TCID50/mL in a 100 dose vaccine preparation calculated from the regression equation. The half-life is the time required for loss of half of the original titre i.e., 0.3 log10TCID50/mL based on the degradation constant. Two factor analyses of variances was performed using SAS 9.2 software to evaluate the effect of stabilizers, level of time, temperatures and their interaction on stability of vaccines in both lyophilized and reconstituted condition. Statistical analysis was carried out using the ANOVA test at the 5% significance level. Wherever ANOVA was found significant, Tukey's test was performed to compare the treatment means.
PPR Vaccine Viruses and Cell lines
Vaccine stabilizers
Preparation of the PPR vaccines
Lyophilization
Measurement of residual moisture
Thermo-stability of freeze-dried vaccines
Thermo-stability of reconstituted vaccines
Virus titration
Statistical analysis
-
The vaccines were freeze-dried in batches with different stabilizers. Under these freeze-drying conditions, there was a loss of virus titer to a very low degree that was within the acceptable limit. The comparative titers before and after lyophilization with respect to different stabilizers are summarized in Table 1. In order to avoid the personal bias on the visual reading to determine the actual final vaccine titre based on CPE, cell-ELISA was carried out to confirm the virus titre in all the vaccine batches.

Table 1. Comparative quality of Thermo-adapted PPR Jhansi/2003 and PPR Revati/2006 vaccines stabilized with LS* and Stabilizer E*
Lyophilization of the vaccine using stabilizer E resulted in the loss of 0.19 to 0.45-log10TCID50/mL titer. The loss in titer for the vaccine stabilized with stabilizer LS ranged from 0.14 to 0.3 log10TCID50/mL (Table 1). Trehalose dihydrate (TD) is one of the best natural cryoprotectants in a dehydrating environment and has been used for dehydration of PPR vaccine without the need for freezing during lyophilization or for desiccation under vacuum conditions [25]. In this study, TD was used for lyophilization of the vaccine in combination with amino acids and divalent cations. In the process of lyophilization, TD dries and forms as a transparent glass resulting in vitrification, which prevents expansion of fluids, thus preventing cells from disruption [2]. The lyophilization behavior and the physico-chemical properties of the freeze-dried products are influenced by the presence of salts in the formulations [8, 9, 23]. Residual moisture (RM) in a vaccine also contributes to its quality [9, 16]. In the present study, RM ranged from 5.3 to 7.22 % (Table 1), which is considered to be relatively high. This may be due to absence of a heating phase of the vaccine vials during the secondary drying process as reported earlier by using conventional freeze-drying techniques [16]. By using the latest of freeze-drying technology, possibly this drawback can be avoided. However, optimum RM content depends on the nature of biological material and on various physical and chemical factors involved in its preparation and dehydration. The higher RM content always has a detrimental effect on the stability of vaccine.
-
The infectivity titres of the freeze-dried vaccines obtained after exposure at 25, 37, 40, 42 and 45℃ for different time intervals were subjected to regression analysis. The result of analysis for each stabilizer at different temperatures is summarized in Table 2.

Table 2. Comparison of degradation values of lyophilized vaccines at various temperatures
At 25℃, stabilizer E showed better shelf-life compared to the LS for both Jhansi and Revati strains. The stabilizer E provided only 30 days thermo-stability for the vaccines, with required infective titre for the protective dose of each vaccine, but did not maintain the required titer for 60 days exposure (Fig. 1 A & B). In general, the data curves followed first-order kinetics, i.e. there is a linear downward trend in titer versus time exposure. Temperatures of 37℃ are normally encountered in many parts of the tropical regions of the country during summer months. Of the stabilizer formulations, stabilizer LS had a relatively superior shelf life (7.62 days) compared to 6.95 days, which was observed with the E stabilizer at 37℃ for the Jhansi strain (Table 2). These shelf life measurements were based on three measurements. However, these differences in shelf life for LS and E are not statistically significant, but only marginal superiority for LS over stabilizer E at 37℃.
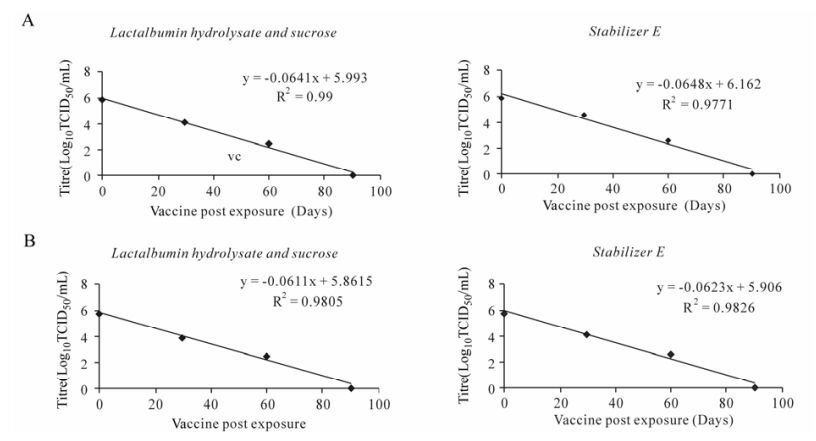
Figure 1. A: Thermo-adapted PPR Jhansi/2003. B: Thermo-adapted PPR Revati/2006. Degradation curves (Regression lines) showing the virus titre at different days exposure at 25℃ with stabilizer LS and Stabilizer E for lyophilized vaccines.
At this temperature, the vaccines stabilized with LS and stabilizer E had initial titers ranging from 5.75 to 5.89 log10TCID50/mL, respectively and if the vaccines were utilized within 7 to 8 days, then the desired 100 doses were found to remain satisfactory with a OIE recommended titer of 2.5 log10TCID50/dose. The overall results indicate that the Ta vaccine strains (Jhansi/2003 and Revati/2006) have a better stability at ambient temperature compared to the current conventional PPR Sungri/96 candidate vaccine [16]. The latter has only 1.58 days & 1.96 days shelf-lives and 17.8 h & 14.07 h of half-lives at 37℃ with LS and TD stabilizers, respectively [16] (Table 2; Fig. 2A & B).
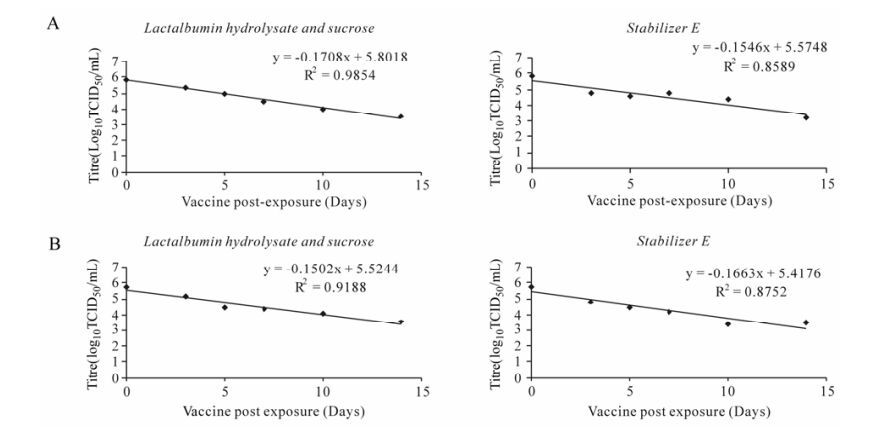
Figure 2. A: Thermo-adapted PPR Jhansi/2003. B: Thermo-adapted PPR Revati /2006. Degradation curves (Regression lines) showing the virus titre at different days exposure at 37℃ with stabilizer LS and Stabilizer E for lyophilized vaccines.
At 40℃, stabilizer LS was found to be marginally superior in terms of shelf-life, whereas, stabilizer E has a better half-life at 40℃ (Table 2, Fig. 3A & B). Similarly at 42℃, the LS stabilizer was found to be advantageous over stabilizer E for PPR Jhansi/2003 vaccine, but the opposite was observed for the half-life. However, there is no statistically signifi-cance differences were observed, which may be due to experimental variation. But, in case of PPR Revati/ 2006 vaccine, both half and shelf-lives were relatively high in stabilizer E with statistically significance difference (Table 2, Fig. 4A & B).
Figure 3. A: Thermo-adapted PPR Jhansi/2003. B: Thermo-adapted PPR Revati/2006. Degradation curves (Regression lines) showing the virus titre at different days exposure at 40℃ with stabilzer LS and Stablizer E for lyophilized vaccines.
Figure 4. A: Thermo-adapted PPR Jhansi/2003. B: Thermo-adapted PPR Revati/2006. Degradation curves (Regression lines) showing the virus titre at different hours exposure at 42℃ with stabilizer LS and Stabilizer E for lyophilized vaccines.
A 45℃ temperature is not uncommon during summer months in several parts of India and other tropical desert countries. At this temperature, both half-and shelf-lives of the vaccines are only a few hours. The half life of the vaccine with stabilizer E was found to be advantageous over LS (Table 2, Fig. 5A & B). With respect to the shelf-lives of the vaccines, stabilizer E was found to have a marginal superiority over the LS stabilizer, which is consistent with an earlier report on the stability of the conventional PPR vaccine when tested with TD and LS [16]. At 45℃ temperature, Ta vaccines had 9.5 to 22.8 h & 24.7 to 27 h shelf-lives and 4.2 to 6.2 h & 8.4 to 12.9 h of halflives with LS and stabilizer E, respectively. Whereas at this temperature, the PPR Sungri/96 vaccine virus with a RM contents of 4.03 to 4.51 % had 5.72 h and 8.11 h of shelf-life and 2.29 h and 1.96 h of half-life only with LS and TD stabilizers, respectively with no detectable titre after 12 and 18 h of exposure of the vaccine [16]. Likewise, the findings shows that the present Ta PPR vaccines formulations with stabilizers used are expected to work better than the previous reported conventional PPR Sungri/96 vaccine at ambient temperatures.
Figure 5. A: Thermo-adapted PPR Jhansi/2003. B: Thermo-adapted PPR Jhansi/2003. Degradation curves (Regression lines) showing the virus titre at different hours exposure at 45℃ with stabilizer LS and Stabilizer E for lyophilized vaccines.
Statistical analysis revealed that there was a significant effect (P < 0.05) in terms of stabilizers, time and temperatures on stability of vaccines in lyophilized conditions. Under these conditions, the effect of stabilizer E in Jhansi strain is statistically (P < 0.05) indistinguishable from the effect of LS and had maximum mean effect on the stability of viruses, whereas Revati strain in LS, which is homogeneous with Revati strain in stabilizer E achieved maximum mean at 42℃ followed by 40, 37, 45 and 25℃ (Table 3). It is clear from the study that the stability of the vaccines was dependent on temperature, length of time. There is no significance (P < 0.05) difference between LS and E stabilizers on the lyophilized vaccines. For the Jhansi strain the stabilizers were equivalent. For the Revati strain the E stabilizer was more effective than stabilizer LS.

Table 3. Comparison of the effect of stabilizers, time and temperatures on stability of vaccines in lyophilized conditions
Earlier studies have indicated that an increase in the concentration of TD may increase the stability of the vaccine [5, 26]. Therefore in the present study, increased concentration of trehalose dihydrate (30%) was used. Sugars stabilize membranes and proteins by hydrogen bonding to the polar residues of the biomolecules, working as a water substitute and the concentrated sugar solution lowers the nucleation temperature of the water inside the virus membrane, thereby preventing large ice crystal formation within both the virus and the external medium. In this study also, the results obtained for stabilizer E were as good as those for the LS stabilizer under conventional freeze-drying conditions. It is worth noting that stabilizer E was found to be a better stabilizer for vaccine used under this study, if, the vaccine is prepared and dehydrated according to Worrall et al. [25]. It has been reported that oral polio vaccine (OPV) vaccine stabilized with 1 mol/L MgSO4can withstand freezing and thawing ten times without any adverse effect on the virus titre and there was no loss in the virus titres for 3 weeks [17]. The successful heat stabilizing ability of a sugar and amino acid combination on the stability of 17-D yellow fever vaccine [1] and Sabin OPV has already been reported [17].
Though Diallo et al. [7]reported the stability of PPR vaccine virus for 14 days at 45℃ with minimal loss of potency, it was observed that the titre came down drastically from 5.2 to < 1.5 after exposure period of 14 days. They used 1% trehalose stabilizer and assessed stability with a freeze-dried vaccine batch (production condition) which had a very low (1.3 %) RM level. But in the present study, the Ta vaccines with LS stabilizer maintained the original titer for 30 h at 40℃ and 2 days at 37℃ without any loss of titre, even though the batch of vaccine had the relatively high RM. So it is logical to speculate that if we can produce a Ta vaccine with minimal moisture, then the stability would be significantly improved.
-
Because the titer of vaccine is likely to decrease over time, the live virus vaccine should be administered as soon as possible after reconstitution so that the animals may receive the required dosage of the vaccine. The survival of viruses is affected by many physical and chemical variables, including temperature, humidity, pH, presence of organic matter, and exposure to various chemicals [5, 8, 24]. The recon stitution was designed with consideration for tropical/ sub-tropical field conditions. The stability of Ta PPR vaccines was examined to determine whether diluted vaccines could be used over an extended period, thereby increasing the number of available doses. Findings in respect of thermo-stability of reconstituted vaccine were quite interesting. Infectivity titre of reconstituted vaccine samples after exposure to various temperatures for each stabilizer is recorded in Table 4.

Table 4. Comparison of stability of vaccines stabilized with LS and Stabilizer E at various temperatures after reconstitution with diluentsa
The vials were taken out from incubator after six hours of exposure because the stability of the reconstituted PPR vaccine up to 6 hours time period has already been reported [16]. It was found that the reconstituted vaccine is capable of withstanding storage at room temperature for a relatively prolonged period with minimal decrease in virus titer. It is somewhat cumbersome to keep reconstituted vaccines in sterile condition in the usual natural environment at field conditions for more than two days, so it was decided not to extend the study beyond 48 h. For the PPR Jhansi/2003 vaccine in stabilizer E at 4℃ and 25℃, there was not much loss in titre even up to 48 h in all the diluents but at 37℃, the diluents 0.85% NaCl and 1 mol/L MgSO4 performed well for a period of 42 h, but thereafter, the titer could not be maintained. Both the diluents performed well for the Jhansi/2003 vaccine in LS stabilizer at 4℃, while at 25℃ best results were obtained for 0.85% NaCl than with 1 mol/L MgSO4, which could maintain the required titre for a period of 30 h. At 37℃, only 0.85% NaCl found to be better for 30 h, while others failed to maintain the same level of stability even for 24 h. Similarly, the Revati/2006 vaccine in stabilizer E fared better in 1 mol/L MgSO4 diluent for 30 h at 4℃ and 24 h at 25℃ as well as at 37℃. The same vaccine with LS stabilizer, 1 mol/L MgSO4was found suitable for 48 h at 4℃ but at 25℃ and 37℃, the stability lasted for 24-30 h. Similar results were noticed with 0.85% NaCl diluent. After statistical analysis it has been found that the reconstituted Jhansi strain in stabilizer E achieved maximum mean rate of virus stability, the Jhansi strain in LS, the Revati strain in LS and Revati strain in stabilizer E all achieved similar values. The vaccines in 0.85% NaCl solution had the maximum mean stability and 0.85% NaCl was performed better and statistically differed 1 mol/L from MgSO4 (P < 0.05) in terms of stability (Table 5). The Jhansi strain with stabilizer E performed better than LS and had significant difference (P < 0.05).

Table 5. Comparison of the effect of diluents on stability of lyophilized vaccines under reconstituted conditions
The Food and Agriculture Organization (FAO) has reported the superiority of 0.85% NaCl and 1 mol/L MgSO4 as diluents for LS-stabilized rinderpest (RP) vaccine [13]. Studies on Vero cell-adapted RP vaccine stabilized with LS-stabilizer by Mariner et al. [9] and Sarkar et al. [16]on PPR vaccine also suggested 1 mol/L MgSO4 as the diluent of choice. Although 0.85% NaCl is not the recommended diluent for PPR vaccine as per Sarkar et al. [16], it was included in these experiments as a possible alternative that could be used in an emergency situation. However, the present study is in slight disagreement with earlier reports and suggests that the vaccine reconstituted with 0.85% NaCl is relatively advantageous over 1 mol/L MgSO4, though most of the times they performed equally well. The vaccine reconstituted with 0.85% NaCl can be used at ambient temperatures even up to 30 h and confirms 0.85% NaCl as the diluent of choice for Ta PPR vaccine. In addition, 0.85% NaCl is more economical than 1 mol/L MgSO4 when bulk production of the vaccine is required [16]. These factors may be considered whenever a PPR control program like the National Program on PPR Eradication (NPPRE) are launched throughout the country [18] or continent-wide in the manner of the RP Eradication program.
Maintaining the vaccines in cold chain is one of the most essential components of a successful immunization program. The study revealed a relatively better stability of Ta vaccines in both the LS and Stabilizer E at ambient temperatures with a relative superiority observed for LS. Vaccines with stabilizer LS was found to maintain the mean titer without any loss for 2 days at 37℃ and at 40℃ even after 30 h for both vaccines. This clearly demonstrated that the vaccine in freeze-dried form is usually stable at ambient temperatures (25℃) for 24-26 days. Once reconstituted in the diluents, the virus titres begin to recede and there is a chance of rapid loss of potency in the field conditions. In these circumstances, Ta PPR vaccines reconstituted with 0.85 % NaCl diluent appear safe to use for 30 h post dilution at ambient temperature, which is normally observed under field conditions, especially in tropical and subtropical countries like India. Furthermore, these experiments, which were conducted to test the thermo-stability, have provided an insight into the storage conditions for Ta PPR vaccines that are usually not recommended in normal circumstances. After extensive field evaluation, we demonstrated that these two vaccines could be used for the control and eradication of the disease in tropical countries like India, avoiding vaccination failure during mass vaccination campaigns due to breakdown in the maintenance of cold-chain, as this vaccine is quiet stable at ambient temperatures.













 DownLoad:
DownLoad: