-
Highly active antiretroviral therapy (HAART) is a combinatorial therapy for HIV-1 infection, using three or more anti-HIV-1 drugs. It has greatly contributed to HIV-1 prevention and treatment since its discovery. However, HIV-1 variants with drug-resistant mutations and decreased drug susceptibility are sometimes found in patients due to frustrated treatment regimens and poor adherence [13]. These variants may set off wider drug resistance transmission, create serious setbacks for HIV-1 therapy [15]. Detection of drug resistance trends, and mutations that facilitate drug resistance, in HIV-1infected patients is therefore critical to the selection of appropriate regimens and efficacious HIV-1 prevention and treatment.
HIV-1 drug resistance detection methods consist of genotypic and phenotypic drug resistance assays. The genotypic drug-resistance assay involves sequencing the HIV-1 viral pol gene in a sample and comparing the sequence to an HIV-1 drug resistance database, such as the Stanford HIV Drug Resistance Database (http://hivdb.stanford.edu/[HIV db]) [4, 12]. Phenotypic drug resistance assays measure the ability of HIV to grow in the presence of different drugs; they are often performed using methods based on peripheral blood monoclonal cells (PBMCs) or polymerase chain reaction (PCR) [5, 6]. Phenotypic drug resistance assays require cells to be cultured, and so are costly and time-consuming. However, phenotypic drug resistance can also be predicted from genotypic data according to the HIV db [8].
Monitoring HIV-1 drug resistance is rarely performed in developing countries because of the cost, but it is essential for effective selection of drugs. The main objective of this study was to evaluate trends of genotypic and predicted phenotypic drug resistance among drug-treated, HIV-1-infected patients in Hubei, China from 2004 to 2006.
HTML
-
A total of 290 drug-treated HIV-1-infected patients in former blood donors in Hubei, China between January 2004 and December 2006 were included in this study. After obtaining patients' consent, pol gene sequencing was performed for genotypic drug-resis tance tests. For each patient, CD4+ cell counts and HIV RNA loads were determined within 3 months of drug-resistance analysis. Characteristics of the 290 patients are shown in Table 1.

Table 1. Characteristics of drug-treated HIV-1-infected patients from 2004 to 2006
-
Nucleotide sequences encoding HIV-1 protease and reverse transcriptase genes (2.1 kb) were amplified from PBMC DNA using a nested PCR method. After TA cloning, DNA sequencing of PCR products was done with an automated ABI 3730 DNA sequencer (Applied Biosystems Inc., USA). Nucleotide sequences thus obtained were screened using the BLAST (National Center for Biotechnology Information, USA) and BioEdit programs (www.mbio.ncsu.edu/BioEdit/bioedit.html) to eliminate potential laboratory errors; some sequences had previously undergone HIV-1 epidemic analysis [10]. Each sample's 2.1 kb sequence was submitted to the HIVdb for genotypic drug resistance analysis.
-
All drug-resistance mutations of each sequence were submitted to the HIVdb for drug resistance predictions. Samples submitted to the HIVdb were identified as having one of five levels of drug resistance: high, intermediate, low, potential low and susceptible. In this study, we labeled high resistance as H, intermediate resistance as I, and low resistance and potential low level resistance as L.
-
Changes in percentages of drug resistance mutations from 2004 to 2006 were analyzed using a Χ2 test. Results in which P > 0.05 were considered insignificant; P < 0.05 was considered significant; and P < 0.01 was considered highly significant. Analyses were performed using the SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL).
Patients
Genotypic drug resistance analysis
Interpretation of genotypic drug resistance
Statistics
-
As shown in Table 2, from 2004 to 2006, we found highly significant increases in percentages of drug-treated patients carrying HIV-1 with either an nucleoside reverse-transcriptase inhibitor (NRTI) or nonnucleoside reverse-transcriptase inhibitor (NNRTI), both an NRTI and an NNRTI, any NRTI, ≥ 2 NRTIs, any NNRTI, ≥ 2 NNRTIs, any thymidine analogue mutation (TAM), or ≥ 2 TAMs.

Table 2. Drug resistance profiles
Over the study period (2004–2006), we found highly significant increases in percentages of drug-treated patients carrying HIV-1 variants with M41L, T215Y/F, D67N, K103N, G190A/S or Y181C/F mutations, significant increase in the percentage of drug-treated patients carrying HIV-1 with L210W, but no significant increases in percentages of drug-treated patients carrying HIV-1 with F116Y, M184V, K219Q/E, Q151M, K70R, K101E/P, V179D, Y188L/C, A98G or V106A mutation (Fig. 1).

Figure 1. Emerging trends of HIV-1 nucleoside reverse transcriptase inhibitors-resistance and non-nucleoside reverse transcriptase inhibitors-resistance mutations. The y-axis shows the percentages of HIV-infected patients carrying each drug-resistant mutation in each year. The x-axis shows names of mutations. Trends in changes in the percentage of each mutation in 2004, 2005 and 2006 were analyzed by Χ2test.
Of the NRTI-resistance mutations, patients with the M41L mutation increased from 1/108 (0.9%) in 2004 to 2/134 (1.5%) in 2005, and to 12/48 (25%) in 2006., Variants with T215Y/F increased fastest, from 1/108 (0.9%) in 2004, to 9/134 (6.7%) in 2005, and to 18/48 (37.5%) in 2006 (P < 0.01). Patients with the L210W mutation increased from 1/108 (0.9%) in 2004, to 3/134 (2.2%) in 2005, and to 4/48 (8.3%) in 2006 (0.01 < P < 0.05). Those with the D67N mutation were first noticed in 2005, 5/134 (3.7%); they increased to 7/48 (14.6%) in 2006 (P < 0.01).
Of the NNRTI-resistance mutations, K103N was the most abundant; it increased from 7/108 (6.5%) in 2004, to 17/134 (12.7%) in 2005, and to 16/48 (33.3%) in 2006 (P < 0.01). The G190A/S mutation increased from 1/108 (0.9%) in 2004, to 7/134 (5.2%) in 2005, and to 7/48 (14.6%) in 2006 (P < 0.01). The Y181C/F variant initially emerged in 2005 at 3/134 (2.2%), and increased to 8/48 (16.7%) in 2006 (P < 0.01).
-
To further analyze phenotypic drug resistance of each HIV-1infected drug-treated patient to NNRTIs and NRTIs, we predicted the phenotypic drug resistance of each patient using the HIVdb. As shown in Fig. 2 and Fig. 3, we found highly significant increases in percentages of HIV-1-infected drug-treated patients carrying high resistance to zidovudine (AZT) or stavudine (D4T) in NRTIs, and to delavirdine (DLV), efavirenz (EFV) or nevirapine (NVP) in NNRTIs; highly significant increases in percentages of HIV-1-infected drug-treated patients carrying intermediate resistance to abacavir (ABC), AZT, D4T, didanosine (DDI) or tenofovir disoproxil fumarate (TDF) in NRTIs, and to etravirine (ETR) in NNRTIs; and highly significant increases in the percentages of HIV-1-infected drug-treated patients carrying low and potentially low resistance to lamivudine (3TC), ABC, emtricitabine (FTC) or TDF in NRTIs, and to ETR in NNRTIs.
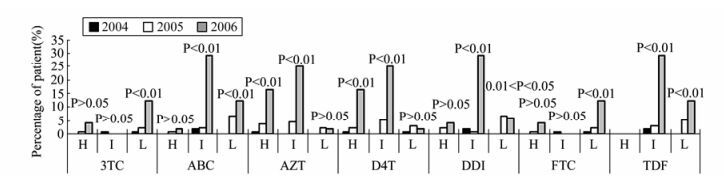
Figure 2. Emerging trends of predicted phenotypic drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs). The y-axis shows the percentages of HIV-infected patients carrying each drug-resistance mutation in each year. The x-axis shows the names of NRTIs and the levels of resistance to the NRTIs: high resistance (H), intermediate resistance (I), or low and potentially low resistance (L). Trends in changes in the percentage of each mutation in 2004, 2005 and 2006 were analyzed by Χ2test. 3TC: lamivudine; ABC: abacavir; AZT: zidovudine; D4T: stavudine; DDI: didanosine; FTC: emtricitabine; TDF: tenofovir disoproxil fumarate.
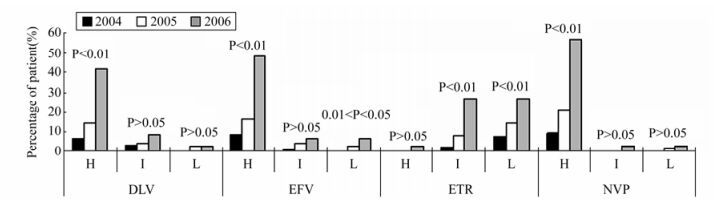
Figure 3. Emerging trends of predicted phenotypic drug resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs). The y-axis shows the percentages of patients harboring the predicted levels of resistance to the NNRTIs in each year. The x-axis shows the name of each NNRTIs and the levels of resistance to the NNRTIs. The levels of resistance are defined as high-level resistance (H), intermediate-level resistance (I), low-level resistance and potentially low-level resistance (L). Trends in changes in the percentage of each mutation in 2004, 2005 and 2006 were analyzed by Χ2test. DLV: delavirdine; EFV: efaviren; ETR: etravirine; NVP: nevirapine.
For predicted NRTI-related phenotypic drug resistance, we only found highly significant increases in patients carrying HIV-1 variants with high resistance to AZT or D4T, which increased from 1/108 (0.9%) or 1/108 (0.9%) in 2004, to 5/134 (3.7%) or 3/134 (2.2%) in 2005, and to 8/48 (16.7%) or 8/48 (16.7%) in 2006, respectively (P < 0.01). For predicted NNRTI-related drug resistance, we found highly significant increases in patients carrying HIV-1 variants with high resistance to DLV, EFV or NVP—from 7/108 (6.5%), 9/108 (8.3%) or 10/108 (9.3%) in 2004, to 19/134 (14.2%), 22/134 (16.4%) or 28/134 (20.9%) in 2005, and to 20/48 (41.7%), 23/48 (47.9%) or 27/48 (56.3%) in 2006, respectively (P < 0.01).
-
Among treated patients, no significant differences in age, gender or drug regimens were seen between those with drug-resistant variants and those with wild-type HIV-1 viruses (data not shown). However, we found a significant difference in HIV-1 viral RNA loads between those with drug -resistant variants and those with wild-type HIV-1 viruses; patients with high HIV-1 RNA loads are prone to carry drug-resistant variants (data not shown).
Emerging trends of HIV-1 drug-resistance muta tions in drug-treated patients
Emerging trends of predicted phenotypic drug resistance to NNRTIs and NRTIs
Statistical analysis
-
In 2003, HAART therapy for HIV-1 became available in Hubei, China. By 2008, 25 anti-HIV drugs had been approved by the United States Food and Drug Administration (FDA) for clinical use in the treatment of AIDS. These include eight NRTIs, four NNRTIs, ten protease inhibitors (PIs), one fusion inhibitor (FI), one cell receptor inhibitor (CRI) and one integrase inhibitor (INI) [3]. However, until December 2006, only six anti-HIV drugs from two categories were available in Hubei, China. These included four NRTIs (AZT, DDI, D4T and 3TC) and two NNRTIs (NVP and EFV). Among them, 3TC was first used in year 2005, and the other five drugs were frequently used from 2004 to 2006. Therefore, in our study, the PI-resistant mutation was sporadic and showed no significant change from 2004 to 2006 (data not shown). We only analyzed resistance to NRTIs and NNRTIs in this paper. The limited drug selection gave rise to rapid increases in drug resistance mutations.
On the other hand, a previous report also showed that drug resistance was much higher in patients who were infected with subtype B than in those infected with non-B viruses [14]. However, subtype B HIV-1 is the predominant subtype in Hubei, China. In the 290 patients we collected in this experiment, more than 95% of patients were infected with subtype B HIV-1. This may also help to explain the rapid increases in drug resistance mutations observed in Hubei, China.
All of the isolates we collected belong to subtype B' HIV-1. Of NRTI-associated genotypic drug-resistance mutations, M41L, T215Y/F, D67N and L210W are the only four mutations that had highly significant or significant increases from 2004 to 2006. They are all types of thymidine analog mutations (TAM), and are selected by the thymidine analogs AZT and D4T [7]. This is consistent with the common use of AZT and D4T in HARRT regimens used to treat HIV-1-infected patients in Hubei since 2003. Presence of TAMs is associated with cross-resistance to many FDA-approved NRTIs [2, 11]. During the study period, in our phenotypic NRTI-resistance predictions, we found highly significant increases in percentages of HIV-1-infected drug-treated patients with high and intermediate resistance to AZT or D4T, and intermediate resistance to ABC, DDI or TDF. These results are consistent with drug regimens used in Hubei until 2006. This suggests that AIDS drug regimens should be changed in the future.
From the NNRTI-associated genotypic drug resistance analyses, we found highly significant increases in K103N, G190A/S and Y181C/F during the study period. Of these, K103N was the most abundant and fast-growing variant in our samples; it alone brought cross-resistance to all of the currently FDA-approved NNRTIs other than ETR [1]. As K103N can produce high-level EFV resistance, and Y181C along with G190A/S etc. can produce resistance to ETR [9], our NNRTI-related drug resistance predictions indicated highly significant increases in percentages and numbers of patients carrying HIV-1 with high resistance to EFV, NVP or DLV, and variants with intermediate level resistance to ETR. These results are consistent with the common use of EFV, NVP in HAART regimens for HIV-1infected patients in Hubei since 2003.
In summary, we found significantly increased genotypic drug resistance and predicted phenotypic drug resistance in drug-treated HIV-1 infected patients in former blood donors in Hubei, China from 2004 to 2006, which suggests that more anti-HIV drugs and different regimens will be needed for AIDS treatment in Hubei in the future. This article provides valuable information for the treatment and control of AIDS in Hubei, China.













 DownLoad:
DownLoad: